Key points
- 49 states and the District of Columbia (DC) participate in DOSE.
- DOSE syndromic surveillance (SYS) data and DOSE emergency department and inpatient hospitalization discharge (DIS) data provide complementary information to understand nonfatal drug overdose trends in the United States.

DOSE helps prevent drug overdose
DOSE uses multiple data sources
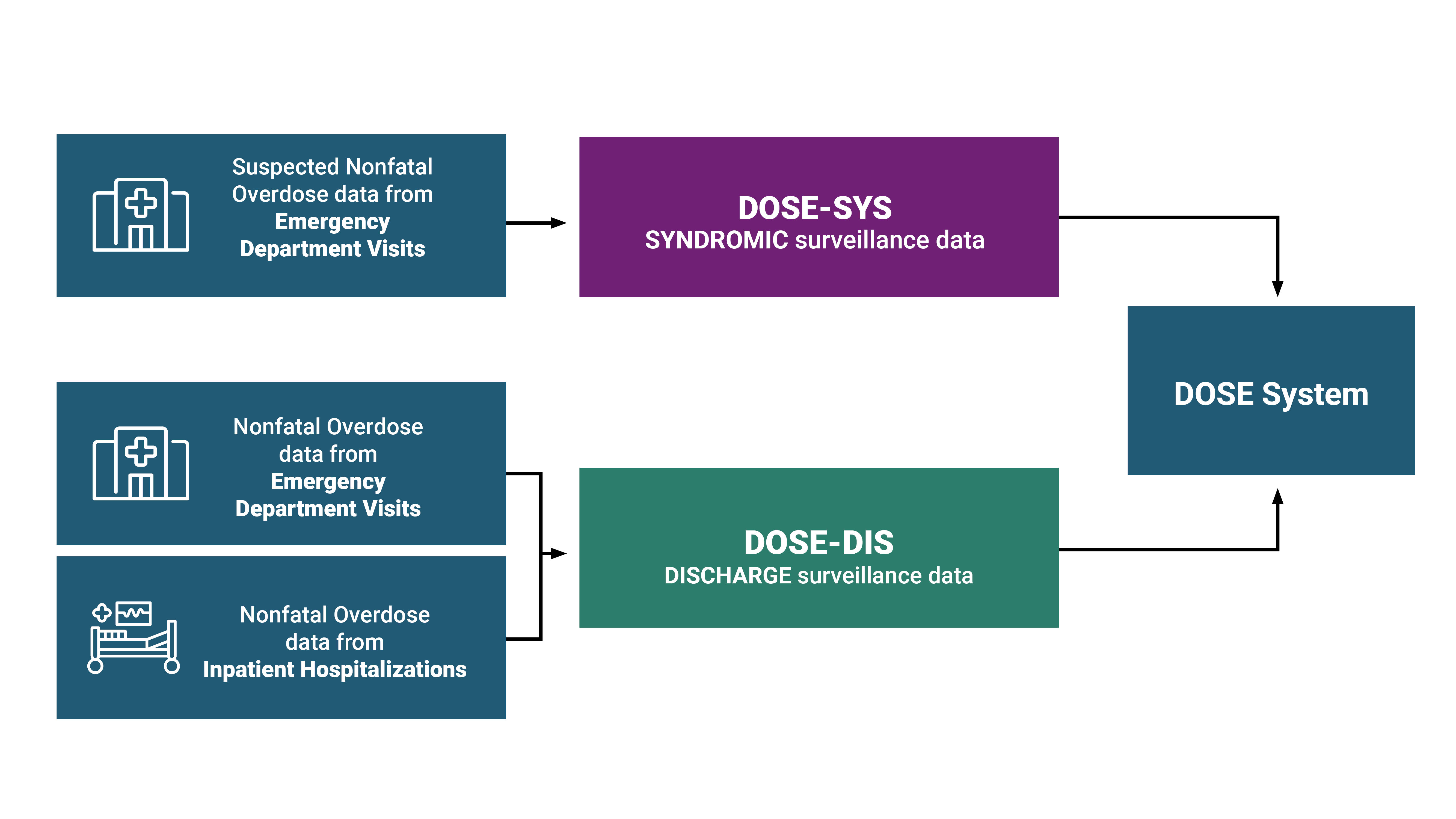
DOSE leverages timely ED syndromic surveillance data captured by health departments to rapidly assess trends and maintain situational awareness of changes in suspected nonfatal drug overdose-related ED visits at the state and national level. On average, National Syndromic Surveillance Program (NSSP) covers about 80% of ED facilities across the United States.
Jurisdictions also share the total number of ED visits for use as the denominator in rate calculations for suspected nonfatal all drug, benzodiazepine-, cocaine-, fentanyl-, heroin-, methamphetamine-, all opioid-, and all stimulant-involved overdoses with CDC with a two-month delay. Diagnosis codes in syndromic surveillance data may be preliminary. In addition, suspected overdoses in syndromic data may not be confirmed by toxicological testing, which is often limited in EDs. Data are disaggregated by 1) age group, sex, race and ethnicity, and 2) county of patient residence. The number of jurisdictions included in the calculations of monthly and annual percent change estimates in rates varies over time. Additionally, comparisons between jurisdictions should not be made because of variations in data quality, completeness, and reporting across jurisdictions. Although coverage is high within participating states (on average >80% of ED facilities in participating states), these data are not nationally representative.
SYNDROMIC SURVEILLANCE DEFINITIONS
The DOSE system uses standardized syndromic surveillance definitions for suspected nonfatal all drug, benzodiazepine-, cocaine-, fentanyl-, heroin-, methamphetamine-, all opioid-, and all stimulant-involved overdoses established by CDC. All definitions draw from multiple fields within ED data to classify visits as overdose-related.
Suspected nonfatal overdose-related ED visits are identified according to diagnosis codes used for clinical diagnosis and insurance billing purposes, specifically, International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and SNOMED CT (Systematized Nomenclature of Medicine — Clinical Terms) codes. Where manner of injury is defined, only diagnosis codes for "unintentional" or "undetermined" intent drug poisoning are included in the DOSE standardized syndromic surveillance definitions.
Although many diagnosis codes provide sufficient indication of a suspected drug overdose, other diagnosis codes specific to opioid drug use, abuse, and dependence, without a mention of intoxication, are less specific (e.g., F11.10: Opioid abuse, uncomplicated); therefore, when these are the only codes present, additional information (e.g., chief complaint field) is needed for the ED visit to be captured as a suspected opioid-involved overdose.
Syndromic surveillance definitions also utilize the free text field called "chief complaint", which represents the purpose of an ED visit, for example, "Patient was found unresponsive. Emergency medical services (EMS) provided Narcan and patient said took heroin." The amount and type of information provided in unstandardized text such as the chief complaint field can vary. To be included as an opioid overdose based solely on chief complaint, the field must include a naloxone term such as "naloxone" or "Narcan." For records not including naloxone terms, records must include two components: 1) text indicating an overdose or poisoning and 2) text indicating the involvement of a drug or a diagnosis code for opioid use, abuse, and dependence without intoxication. The free text search of the chief complaint field is not case sensitive and common misspellings of key search terms, for example, "herion" instead of "heroin", are also included. A list of exclusions is applied to the chief complaint field after all inclusion criteria have been assessed. For example, if a diagnosis code for heroin-involved poisoning is not present, exclusions such as "no loss of consciousness", "denied heroin", "detox", and "withdrawal" would be applied and therefore the visit would not be captured as a suspected heroin-involved overdose.
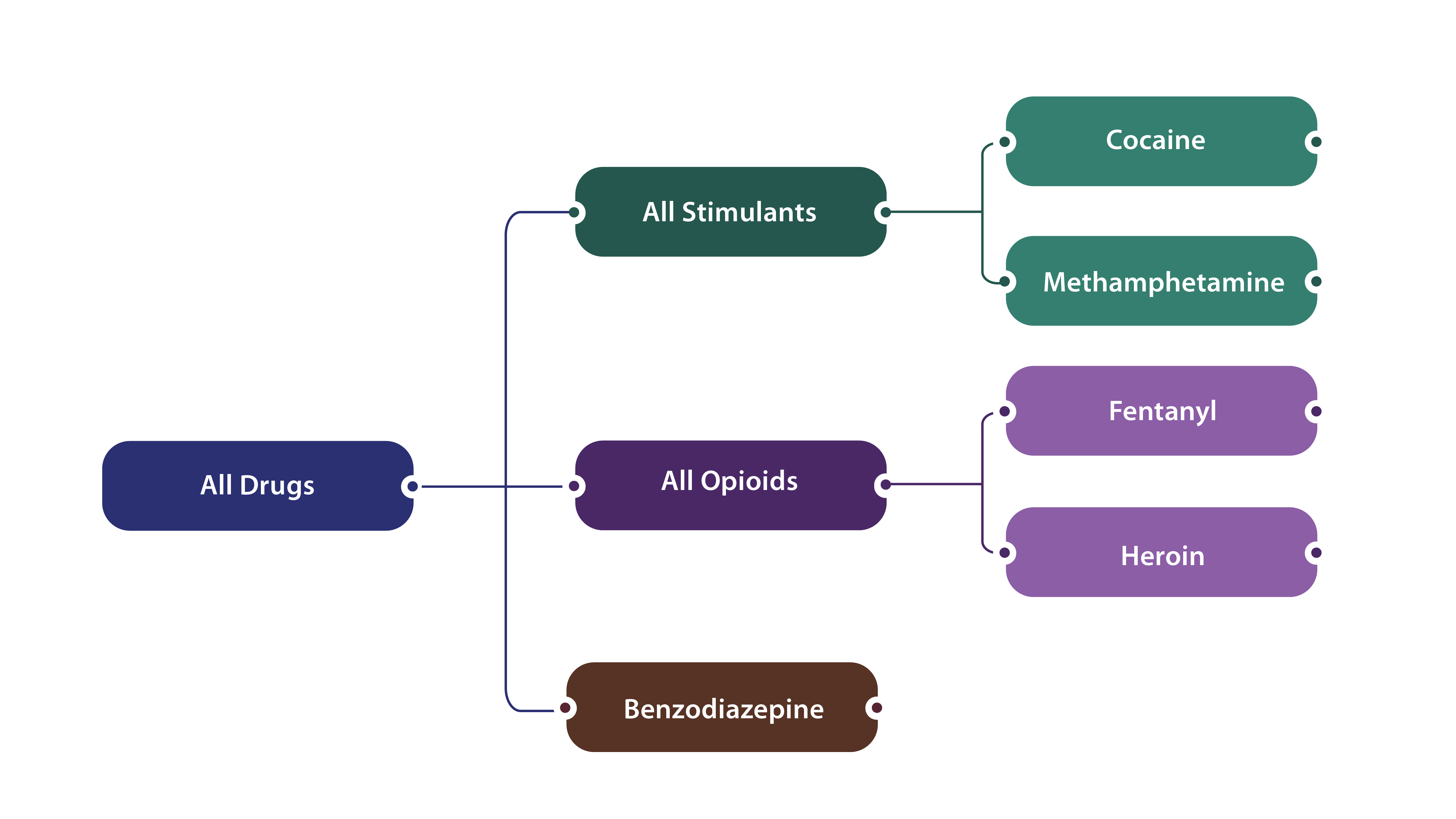
The syndromic surveillance definitions are not mutually exclusive but rather reflect nesting of drug categories (depicted in the figure below) and some overdose visits may involve multiple substances (e.g., a given overdose-related ED visit could have involved both opioids and stimulants).
Please click on each link below to see the current full nonfatal syndromic surveillance definition:
DOSE leverages finalized discharge data from ED visits and inpatient hospitalizations to estimate trends in nonfatal overdoses and calculate burden. Discharge data offer a more complete and accurate understanding of overdose burden compared to syndromic surveillance data. Currently, 34 states and DC share discharge data with DOSE: 31 states and DC share both ED and inpatient hospitalization discharge data, no states share only ED discharge data, and 3 states share only inpatient hospitalization discharge data.
Participating jurisdictions share aggregate data on total ED visits and inpatient hospitalizations and line-level data on nonfatal overdoses with CDC annually, with a 6-month lag. Whereas syndromic surveillance data are preliminary and may not contain a confirmed diagnosis, discharge data may offer a more complete and accurate estimation of overdose burden in EDs and among hospitalizations. Collected data include patient-level information on age, sex, race, ethnicity, and county of patient residence.
ED and inpatient hospitalization discharge data are collected for administrative/billing purposes. The standardized coding scheme is considered more reliable than self-reports, and less expensive to obtain than survey data or medical chart abstractions. However, billing coders assign diagnosis codes in discharge data and the assigned codes may not provide a fully accurate, comprehensive account of the conditions treated. Diagnosis codes may be reported strategically to optimize reimbursement, and surveillance for drug overdoses using these data may not accurately reflect the true overdose burden.
Additionally, the overdoses identified may not be confirmed by toxicological testing, which is often limited in ED or hospital settings. Data not available by the reporting deadline may not ever be submitted, as data are typically considered final at submission. Comparisons between ED and inpatient hospitalization discharge data should be made with caution, as some states submit ED data only or inpatient hospitalization data only. Although coverage is high within participating states (on average >90% of ED facilities in participating states), these data are not nationally representative.
DISCHARGE DATA DEFINITIONS
Standardized discharge data definitions for categorizing nonfatal all drug, all opioid-, heroin-, fentanyl- benzodiazepine-, all stimulant-, methamphetamine-, and cocaine-involved overdoses are established by CDC. While syndromic surveillance definitions are applied to the chief complaint and diagnosis codes available immediately following a patient encounter, discharge data definitions are applied to ICD-10-CM codes reported in finalized medical billing data discharge diagnosis fields. Jurisdictions submit line-level data on visits involving any T36-T50 ICD-10-CM codes to CDC, which then applies DOSE discharge data definitions to identify initial encounters for nonfatal drug overdoses of unintentional/undetermined intent.
While syndromic surveillance definitions are applied to the chief complaint and discharge diagnosis codes available immediately following a patient encounter, discharge data definitions are applied to ICD-10-CM codes reported in finalized medical billing data discharge diagnosis fields. Discharge data definitions are applied by submitting jurisdictions to identify initial encounters for acute drug poisonings.
Similar to the syndromic surveillance definitions, the discharge data definitions are nested.

Additionally, some overdose visits may involve multiple substances (e.g., a given overdose-related ED visit or inpatient hospitalization could have involved both opioids and stimulants, so the single visit would count towards both drug types).
The tables below detail the ICD-10-CM codes used for identifying initial encounters for nonfatal overdoses of unintentional and undetermined intent using DOSE discharge data. It is important to note that new ICD-10-CM codes, such as those for emerging drugs, may be added to the case definitions as diagnosis codes evolve. This comprehensive approach ensures that all relevant substances contributing to the progression of the drug overdose epidemic are adequately captured in the data submission process.
All Drug Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T36.0X1A, T36.0X4A, T36.1X1A, T36.1X4A, T36.2X1A, T36.2X4A, T36.3X1A, T36.3X4A, T36.4X1A, T36.4X4A, T36.5X1A, T36.5X4A, T36.6X1A, T36.6X4A, T36.7X1A, T36.7X4A, T36.8X1A, T36.8X4A, T36.91XA, T36.94XA
Poisoning by systemic antibiotics, such as penicillins, cephalosporins and other beta-lactam antibiotics, chloramphenicol group, etc.
T37.0X1A, T37.0X4A, T37.1X1A, T37.1X4A, T37.2X1A, T37.2X4A, T37.3X1A, T37.3X4A, T37.4X1A, T37.4X4A, T37.5X1A, T37.5X4A, T37.8X1A, T37.8X4A, T37.91XA, T37.94XA
Poisoning by other systemic anti-infectives and antiparasitics, such as sulfonamides, antimycobacterial drugs, antimalarials and drugs acting on other blood protozoa, etc.
T38.0X1A, T38.0X4A, T38.1X1A, T38.1X4A, T38.2X1A, T38.2X4A, T38.3X1A, T38.3X4A, T38.4X1A, T38.4X4A, T38.5X1A, T38.5X4A, T38.6X1A, T38.6X4A, T38.7X1A, T38.7X4A, T38.801A, T38.804A, T38.811A, T38.814A, T38.891A, T38.894A, T38.901A, T38.904A, T38.991A, T38.994A
Poisoning by hormones and their synthetic substitutes and antagonists, not elsewhere classified, such as glucocorticoids and synthetic analogues, thyroid hormones and substitutes, antithyroid drugs, etc.
T39.011A, T39.014A, T39.091A, T39.094A, T39.1X1A, T39.1X4A, T39.2X1A, T39.2X4A, T39.311A, T39.314A, T39.391A, T39.394A, T39.4X1A, T39.4X4A, T39.8X1A, T39.8X4A, T39.91XA, T39.94XA
Poisoning by nonopioid analgesics, antipyretics and antirheumatics, such as aspirin, salicylates, 4-Aminophenol derivatives, etc.
T40.0X1A, T40.0X4A, T40.1X1A, T40.1X4A, T40.2X1A, T40.2X4A, T40.3X1A, T40.3X4A, T40.4X1A, T40.4X4A, T40.411A, T40.414A, T40.421A, T40.424A, T40.491A, T40.494A, T40.5X1A, T40.5X4A, T40.601A, T40.604A, T40.691A, T40.694A, T40.7X1A, T40.7X4A, T40.711A, T40.714A, T40.721A, T40.724A, T40.8X1A, T40.8X4A, T40.901A, T40.904A, T40.991A, T40.994A
Poisoning by narcotics and psychodysleptics [hallucinogens], such as fentanyl, heroin, cocaine, cannabis, etc.
T41.0X1A, T41.0X4A, T41.1X1A, T41.1X4A, T41.201A, T41.204A, T41.291A, T41.294A, T41.3X1A, T41.3X4A, T41.41XA, T41.44XA, T41.5X1A, T41.5X4A
Poisoning by anesthetics and therapeutic gases, such as inhaled anesthetics, intravenous anesthetics, unspecified general anesthetics, etc.
T42.0X1A, T42.0X4A, T42.1X1A, T42.1X4A, T42.2X1A, T42.2X4A, T42.3X1A, T42.3X4A, T42.4X1A, T42.4X4A, T42.5X1A, T42.5X4A, T42.6X1A, T42.6X4A, T42.71XA, T42.74XA, T42.8X1A, T42.8X4A
Poisoning by antiepileptic, sedative-hypnotic and antiparkinsonism drugs, such as hydantoin derivatives, iminostilbenes, succinimides and oxazolidinediones, etc.
T43.011A, T43.014A, T43.021A, T43.024A, T43.1X1A, T43.1X4A, T43.201A, T43.204A, T43.211A, T43.214A, T43.221A, T43.224A, T43.291A, T43.294A, T43.3X1A, T43.3X4A, T43.4X1A, T43.4X4A, T43.501A, T43.504A, T43.591A, T43.594A, T43.601A, T43.604A, T43.611A, T43.614A, T43.621A, T43.624A, T43.631A, T43.634A, T43.641A, T43.644A, T43.651A, T43.654A, T43.691A, T43.694A, T43.8X1A, T43.8X4A, T43.91XA, T43.94XA
Poisoning by psychotropic drugs, not elsewhere classified, such as tricyclic antidepressants, methamphetamines, other psychostimulants, etc.
T44.0X1A, T44.0X4A, T44.1X1A, T44.1X4A, T44.2X1A, T44.2X4A, T44.3X1A, T44.3X4A, T44.4X1A, T44.4X4A, T44.5X1A, T44.5X4A, T44.6X1A, T44.6X4A, T44.7X1A, T44.7X4A, T44.8X1A, T44.8X4A, T44.901A, T44.904A, T44.991A, T44.994A
Poisoning by drugs primarily affecting the autonomic nervous system, such as anticholinesterase agents, other parasympathomimetics [cholinergics], ganglionic blocking drugs, etc.
T45.0X1A, T45.0X4A, T45.1X1A, T45.1X4A, T45.2X1A, T45.2X4A, T45.3X1A, T45.3X4A, T45.4X1A, T45.4X4A, T45.511A, T45.514A, T45.521A, T45.524A, T45.601A, T45.604A, T45.611A, T45.614A, T45.621A, T45.624A, T45.691A, T45.694A, T45.7X1A, T45.7X4A, T45.8X1A, T45.8X4A, T45.91XA, T45.94XA
Poisoning by primarily systemic and hematological agents, not elsewhere classified, such as antiallergic and antiemetic drugs, antineoplastic and immunosuppressive drugs, vitamins, etc.
T46.0X1A, T46.0X4A, T46.1X1A, T46.1X4A, T46.2X1A, T46.2X4A, T46.3X1A, T46.3X4A, T46.4X1A, T46.4X4A, T46.5X1A, T46.5X4A, T46.6X1A, T46.6X4A, T46.7X1A, T46.7X4A, T46.8X1A, T46.8X4A, T46.901A, T46.904A, T46.991A, T46.994A
Poisoning by agents primarily affecting the cardiovascular system, such as cardiac-stimulant glycosides and drugs of similar action, calcium-channel blockers, other antidysrhythmic drugs, etc.
T47.0X1A, T47.0X4A, T47.1X1A, T47.1X4A, T47.2X1A, T47.2X4A, T47.3X1A, T47.3X4A, T47.4X1A, T47.4X4A, T47.5X1A, T47.5X4A, T47.6X1A, T47.6X4A, T47.7X1A, T47.7X4A, T47.8X1A, T47.8X4A, T47.91XA, T47.94XA
Poisoning by agents primarily affecting the gastrointestinal system, such as histamine H2-receptor blockers, other antacids and anti-gastric-secretion drugs, stimulant laxatives, etc.
T48.0X1A, T48.0X4A, T48.1X1A, T48.1X4A, T48.201A, T48.204A, T48.291A, T48.294A, T48.3X1A, T48.3X4A, T48.4X1A, T48.4X4A, T48.5X1A, T48.5X4A, T48.6X1A, T48.6X4A, T48.901A, T48.904A, T48.991A, T48.994A
Poisoning by agents primarily acting on smooth and skeletal muscles and the respiratory system, such as oxytocic drugs, skeletal muscle relaxants [neuromuscular blocking agents], unspecified drugs acting on muscles, etc.
T49.0X1A, T49.0X4A, T49.1X1A, T49.1X4A, T49.2X1A, T49.2X4A, T49.3X1A, T49.3X4A, T49.4X1A, T49.4X4A, T49.5X1A, T49.5X4A, T49.6X1A, T49.6X4A, T49.7X1A, T49.7X4A, T49.8X1A, T49.8X4A, T49.91XA, T49.94XA
Poisoning by topical agents primarily affecting skin and mucous membrane and by ophthalmological, otorhinorlaryngological and dental drugs, such as local antifungal, anti-infective and anti-inflammatory drugs, antipruritics, local astringents and local detergents, etc.
T50.0X1A, T50.0X4A, T50.1X1A, T50.1X4A, T50.2X1A, T50.2X4A, T50.3X1A, T50.3X4A, T50.4X1A, T50.4X4A, T50.5X1A, T50.5X4A, T50.6X1A, T50.6X4A, T50.7X1A, T50.7X4A, T50.8X1A, T50.8X4A, T50.901A, T50.904A, T50.911A, T50.914A, T50.991A, T50.994A, T50.A11A, T50.A14A, T50.A21A, T50.A24A, T50.A91A, T50.A94A, T50.B11A, T50.B14A, T50.B91A, T50.B94A, T50.Z11A, T50.Z14A, T50.Z91A, T50.Z94A
Poisoning by diuretics and other and unspecified drugs, medicaments and biological substances, such as mineralocorticoids and their antagonists, loop [high-ceiling] diuretics, carbonic-anhydrase inhibitors, benzothiadiazides and other diuretics, etc.
Benzodiazepine Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T42.4X1A
Poisoning by benzodiazepines, accidental (unintentional), initial encounter.
T42.4X4A
Poisoning by benzodiazepines, undetermined, initial encounter.
All Opioid Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T40.0X1A
Poisoning by opium, accidental (unintentional), initial encounter.
T40.0X4A
Poisoning by opium, undetermined, initial encounter.
T40.1X1A
Poisoning by heroin, accidental (unintentional), initial encounter.
T40.1X4A
Poisoning by heroin, undetermined, initial encounter.
T40.2X1A
Poisoning by other opioids, accidental (unintentional), initial encounter.
T40.2X4A
Poisoning by other opioids, undetermined, initial encounter.
T40.3X1A
Poisoning by methadone, accidental (unintentional), initial encounter.
T40.3X4A
Poisoning by methadone, undetermined, initial encounter.
T40.4X1A
Poisoning by other synthetic narcotics, accidental (unintentional), initial encounter.
[This code was replaced by T40.411A, T40.421A, and T40.491A beginning October 1, 2020.]
T40.4X4A
Poisoning by other synthetic narcotics, undetermined, initial encounter.
[This code was replaced by T40.414A, T40.424A, and T40.494A beginning October 1, 2020.]
T40.411A
Poisoning by fentanyl or fentanyl analogs, accidental (unintentional), initial encounter.
T40.414A
Poisoning by fentanyl or fentanyl analogs, undetermined, initial encounter.
T40.421A
Poisoning by tramadol, accidental (unintentional), initial encounter.
T40.424A
Poisoning by tramadol, undetermined, initial encounter.
T40.491A
Poisoning by other synthetic narcotics, accidental (unintentional), initial encounter.
T40.494A
Poisoning by other synthetic narcotics, undetermined, initial encounter.
T40.601A
Poisoning by unspecified narcotics, accidental (unintentional), initial encounter.
T40.604A
Poisoning by unspecified narcotics, undetermined, initial encounter.
T40.691A
Poisoning by other narcotics, accidental (unintentional), initial encounter.
T40.694A
Poisoning by other narcotics, undetermined, initial encounter.
Fentanyl Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T40.411A
Poisoning by fentanyl or fentanyl analogs, accidental (unintentional), initial encounter.
T40.414A
Poisoning by fentanyl or fentanyl analogs, undetermined, initial encounter.
Heroin Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T40.1X1A
Poisoning by heroin, accidental (unintentional), initial encounter.
T40.1X4A
Poisoning by heroin, undetermined, initial encounter.
All Stimulant Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T40.5X1A
Poisoning by cocaine, accidental (unintentional), initial encounter.
T40.5X4A
Poisoning by cocaine, undetermined, initial encounter.
T43.601A
Poisoning by unspecified psychostimulants, accidental (unintentional), initial encounter.
T43.604A
Poisoning by unspecified psychostimulants, undetermined, initial encounter.
T43.611A
Poisoning by caffeine, accidental (unintentional), initial encounter.
T43.614A
Poisoning by caffeine, undetermined, initial encounter.
T43.621A
Poisoning by amphetamines, accidental (unintentional), initial encounter.
T43.624A
Poisoning by amphetamines, undetermined, initial encounter.
T43.631A
Poisoning by methylphenidate, accidental (unintentional), initial encounter.
T43.634A
Poisoning by methylphenidate, undetermined, initial encounter.
T43.641A
Poisoning by ecstasy, accidental (unintentional), initial encounter.
T43.644A
Poisoning by ecstasy, undetermined, initial encounter.
T43.651A
Poisoning by methamphetamines, accidental (unintentional), initial encounter.
T43.654A
Poisoning by methamphetamines, undetermined, initial encounter.
T43.691A
Poisoning by other psychostimulants, accidental (unintentional), initial encounter.
T43.694A
Poisoning by other psychostimulants, undetermined, initial encounter.
Cocaine Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T40.5X1A
Poisoning by cocaine, accidental (unintentional), initial encounter.
T40.5X4A
Poisoning by cocaine, undetermined, initial encounter.
Methamphetamine Overdose Discharge Data Definition1
ICD-10-CM for unintentional or undetermined intent
Description
T43.651A
Poisoning by methamphetamines, accidental (unintentional), initial encounter.
T43.654A
Poisoning by methamphetamines, undetermined, initial encounter.
1 ICD-10-CM codes included in discharge data definitions are limited to those that indicated accidental (unintentional) or undetermined intent and indicated an initial encounter for the drug poisoning. Codes that indicated adverse effect of or underdosing are not included in DOSE discharge data case definitions.
DOSE collects nonfatal overdose data
Archived data reports
The following four data reports come from CDC's Enhanced State Opioid Overdose Surveillance (ESOOS) program, which was funded from 2016-2019 to provide more timely and comprehensive data on fatal and nonfatal opioid overdoses and risk factors associated with fatal overdoses. Twelve states were initially funded in September 2016, and an additional 20 states and the District of Columbia were funded in September 2017, to share data on nonfatal all drug, all opioid, and/or heroin overdoses with CDC on a quarterly basis. Data from ESOOS have not been updated since April 2019, and data may differ from those in the DOSE system. For example, ESOOS states were able to develop and apply their own overdose case definitions, whereas DOSE required standardized case definitions to be used across all funded recipients. Furthermore, stimulant overdose data are reported in the DOSE system but were not collected as part of the ESOOS program.
The data report below comes from CDC's DOSE system. The report displays total ED visits, ED visit counts for suspected overdoses, and overdose rates overall and by 41 states and the District of Columbia, through September 2020. During the first several months of the COVID-19 pandemic, the number of total ED visits across the United States substantially declined; however, nonfatal overdoses did not decline at a similar pace.
There are several important caveats to consider when viewing the figures included in this report. During the COVID-19 pandemic, states onboarded new facilities that began sharing data; also, some facilities stopped sharing data during this period. Thus, the number of facilities included was not constant over this time period. In addition, states collaborated with existing facilities to increase the proportion of ED visits that contained diagnosis codes, which facilitates the identification of overdose-related visits. Caution is warranted in interpreting counts, rates, and comparisons over time due to these data issues.