Key points
- 49 states and the District of Columbia (DC) participate in DOSE.
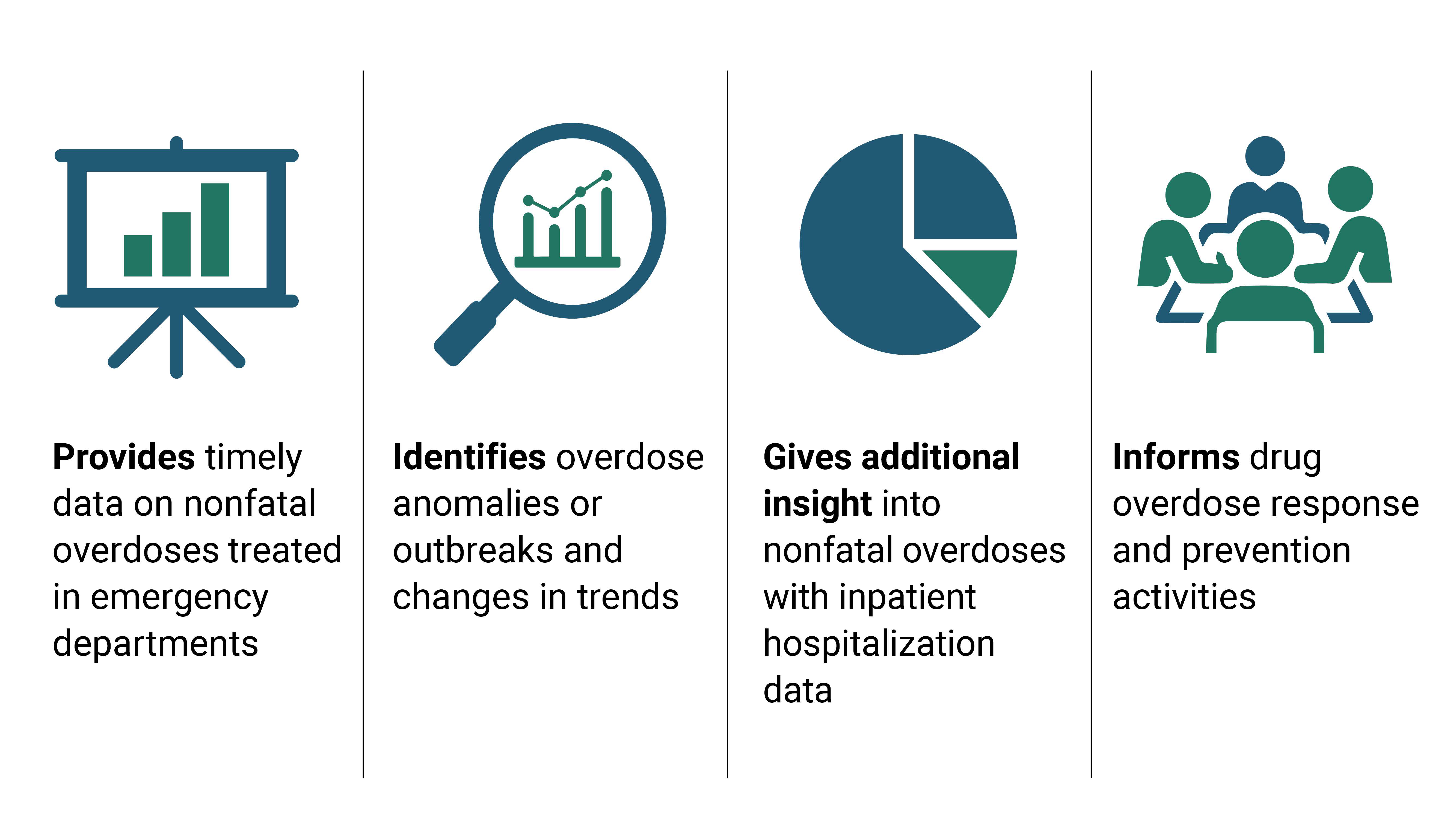
- DOSE syndromic surveillance (SYS) data and DOSE emergency department and inpatient hospitalization discharge (DIS) data provide complementary information to understand nonfatal drug overdose trends in the United States.

DOSE helps prevent drug overdose
DOSE uses multiple data sources
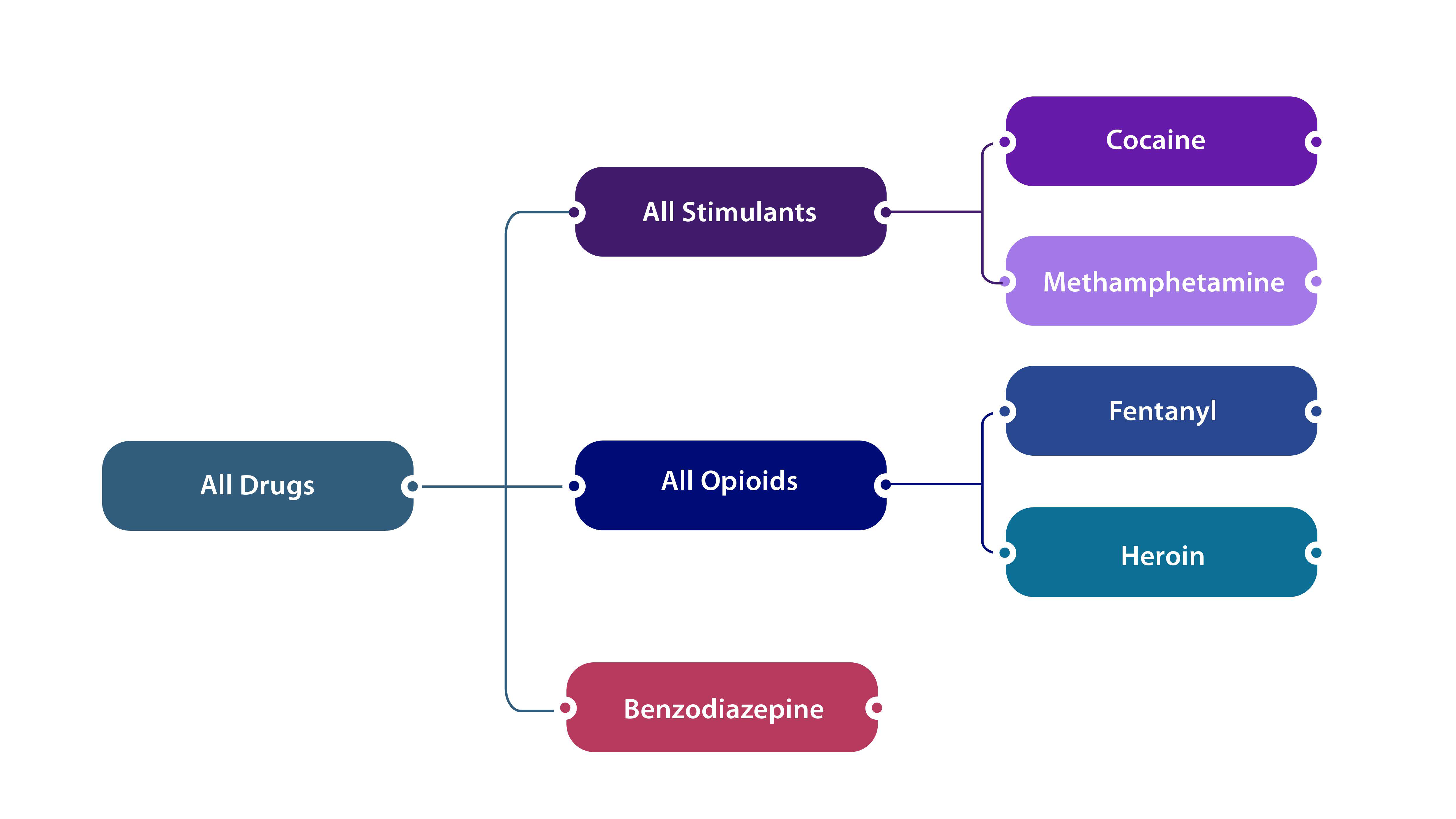
The DOSE system uses standardized surveillance definitions established by CDC for nonfatal overdoses of unintentional or undetermined intent involving all drugs, all opioids, fentanyl, heroin, all stimulants, cocaine, methamphetamine, and benzodiazepine. The specifics of the definitions can be further explored on the respective DOSE-SYS and DOSE-DIS dashboards.
In both DOSE-SYS and DOSE-DIS, the surveillance definitions are not mutually exclusive but rather reflect nesting of drug categories (depicted in the figure below). Some overdose visits may involve multiple substances (e.g., a given overdose-related emergency department visit or inpatient hospitalization could have involved both opioids and stimulants).
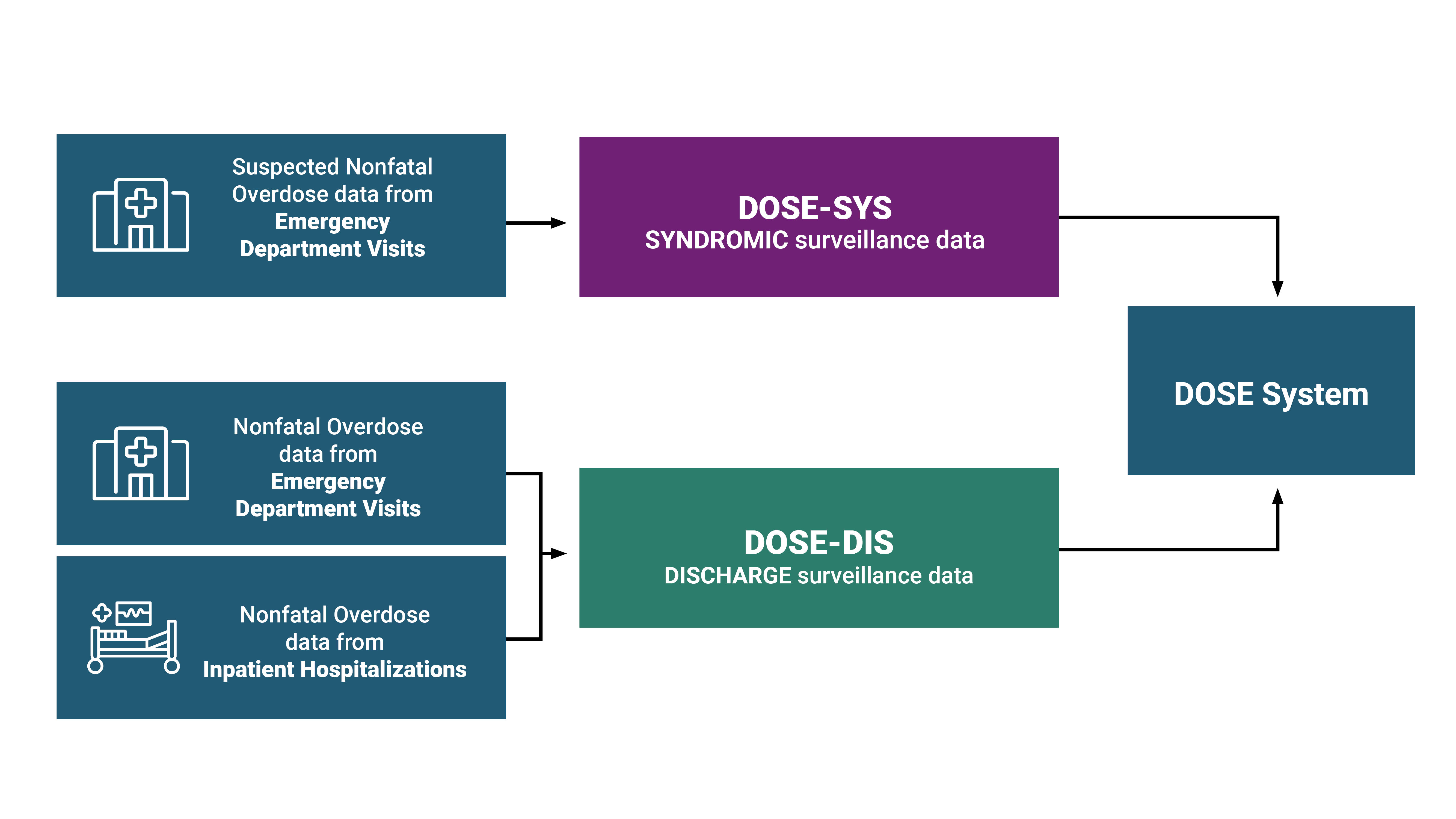
DOSE-SYS leverages timely ED syndromic surveillance data to rapidly assess trends and maintain situational awareness of changes in suspected nonfatal drug overdose-related ED visits at the state and national level. Health departments in forty-six states and DC share syndromic surveillance data with DOSE-SYS. ED visits for suspected nonfatal drug overdose are reported using electronic health record text queries from syndromic surveillance systems. Coverage is high within participating states (on average >80% of ED facilities in participating states). For more information, including details about DOSE-SYS syndrome definitions, please see the DOSE-SYS Dashboard.
DOSE leverages finalized discharge data from ED visits and inpatient hospitalizations to estimate trends in nonfatal overdoses and calculate burden. ED and inpatient hospitalization discharge data are collected for administrative/billing purposes and offer a more complete and accurate understanding of overdose burden compared to syndromic surveillance data. Currently, 34 states and DC share discharge data with DOSE: 31 states and DC share both ED and inpatient hospitalization discharge data, no states share only ED discharge data, and 3 states share only inpatient hospitalization discharge data. Coverage is high within participating states (on average >90% of ED and hospital facilities in participating states). For more information, please visit the DOSE-DIS Dashboard.
DOSE collects nonfatal overdose data
Archived data reports
The following four data reports come from CDC's Enhanced State Opioid Overdose Surveillance (ESOOS) program, which was funded from 2016-2019 to provide more timely and comprehensive data on fatal and nonfatal opioid overdoses and risk factors associated with fatal overdoses. Twelve states were initially funded in September 2016, and an additional 20 states and the District of Columbia were funded in September 2017, to share data on nonfatal all drug, all opioid, and/or heroin overdoses with CDC on a quarterly basis. Data from ESOOS have not been updated since April 2019, and data may differ from those in the DOSE system. For example, ESOOS states were able to develop and apply their own overdose case definitions, whereas DOSE required standardized case definitions to be used across all funded recipients. Furthermore, stimulant overdose data are reported in the DOSE system but were not collected as part of the ESOOS program.
The data report below comes from CDC's DOSE system. The report displays total ED visits, ED visit counts for suspected overdoses, and overdose rates overall and by 41 states and the District of Columbia, through September 2020. During the first several months of the COVID-19 pandemic, the number of total ED visits across the United States substantially declined; however, nonfatal overdoses did not decline at a similar pace.
There are several important caveats to consider when viewing the figures included in this report. During the COVID-19 pandemic, states onboarded new facilities that began sharing data; also, some facilities stopped sharing data during this period. Thus, the number of facilities included was not constant over this time period. In addition, states collaborated with existing facilities to increase the proportion of ED visits that contained diagnosis codes, which facilitates the identification of overdose-related visits. Caution is warranted in interpreting counts, rates, and comparisons over time due to these data issues.
Suggested citation
Centers for Disease Control and Prevention. Nonfatal Drug Overdose Surveillance and Epidemiology (DOSE) System. Atlanta, GA: US Department of Health and Human Services, CDC; [INSERT YEAR, MONTH, DAY]. Access at: https://www.cdc.gov/overdose-prevention/data-research/facts-stats/about-dose-system.html