FAQs: Ventilator-Associated (VAE) Events
- Lower respiratory tract events
- Pneumonia present on admission or prior to initiation of ventilation and VAE surveillance
- Excluded ventilator modes
- Weaning/mechanical ventilation liberation trials and VAE
- Daily minimum values
- PEEP values between 0 cmH2O and 5 cmH2O
- APRV
- Temperature and WBC count
- Antimicrobial agent Appendix
- Antimicrobial agent
- QADs
- Culture results
- Excluded pathogens
- Specimen
- Cytology findings
- Secondary BSI to lower respiratory events in locations performing VAE surveillance
- Secondary BSI pathogens
- Brain injury/ brain death in ventilated patients
- VAE upgrades
- Episode of mechanical ventilation
- Ventilator data
- Home ventilators
- Location of mechanical ventilation
- Date of mechanical ventilation initiation
- VAE specific events
- Benchmarking
Lower respiratory tract events
We know this can be an area of confusion. We need to consider events occurring in patients on mechanical ventilation and events occurring in patients NOT on mechanical ventilation, and we have to consider events that occur in adults and events that occur in neonates and in children. Let’s review what is available for in-plan or off-plan surveillance of lower respiratory tract events in NHSN. Keep in mind that “in-plan” surveillance means that you/your facility have committed to following the NHSN surveillance protocol for that particular event in your NHSN monthly reporting plan. “Off-plan” surveillance is surveillance that is done because you/your facility have decided to track a particular event for internal use. Data that are entered into NHSN “off-plan” are not used or reported in NSHN annual reports or other NHSN publications. A facility makes no commitment to follow the protocol for “off-plan” events.
What lower respiratory tract event surveillance can be done “in-plan”?
- VAE: This event is available for in-plan surveillance of mechanically ventilated inpatients in adult locations only (regardless of the age of the patient). Pediatric and neonatal locations are excluded from VAE surveillance (even in circumstances where a pediatric unit may occasionally care for adult patients).
- PedVAP: This event uses the PNEU protocol and is available for in-plan surveillance of mechanically ventilated inpatients in pediatric locations only (regardless of the age of the patient). In-plan surveillance for ventilator-associated PNEU (VAP) is not available for inpatients in adult or neonatal locations.
- PedVAE: This event is available for in-plan surveillance of mechanically ventilated inpatients in pediatric and neonatal locations only. Adult locations are excluded from PedVAE surveillance (even in circumstances where an adult unit may occasionally care for pediatric patients).
NOTE: When conducting CLABSI surveillance, the PNEU definition is available for use as a site-specific infection to which a bloodstream infection (BSI) can be attributed as a secondary BSI for all patients, all locations, regardless of use of mechanical ventilation. This includes ventilated or non-ventilated adults, children, or neonates in any patient location.
What lower respiratory tract event surveillance can be done “off-plan”?
- VAE: VAE surveillance can be done “off-plan” in mechanically ventilated inpatients in adult locations only.
- PedVAP or PNEU/VAP: Surveillance using the PNEU protocol is available for “off-plan” surveillance in all mechanically ventilated inpatients (adults, children, or neonates) in any inpatient location.
- PedVAE: PedVAE surveillance can be done “off-plan” in mechanically ventilated inpatients in neonatal and pediatric locations only.
- PNEU: PNEU surveillance is available for “off-plan” surveillance in non-mechanically ventilated adults, children, and neonates in any inpatient location.
- LRI: Surveillance for non-pneumonia lower respiratory infections (using LUNG definition) is available for “off-plan” surveillance in mechanically ventilated or non-mechanically ventilated adults, children, and neonates.
Yes. VAE and PNEU/VAP are two separate protocols and detect different types of events within NHSN.
An adult location can conduct simultaneous in-plan VAE surveillance and off-plan PNEU/VAP surveillance, and a patient can meet one and not the other, meet both, or meet neither. In other words, detection of one type of event (such as a VAE) in a particular patient would have no bearing on the conduct of surveillance for the other event type (PNEU/VAP) in the same patient. Patients who meet a VAE definition and a PNEU/VAP definition would have two events identified in units where surveillance for multiple respiratory events is occurring.
Pneumonia present on admission or prior to initiation of ventilation and VAE surveillance
No. Tracking of daily minimum PEEP and FiO2 should be done for all patients who are eligible for VAE surveillance in units in which in-plan VAE surveillance is being conducted, regardless of the reason for which the patient was admitted or the reason for initiation of mechanical ventilation. Additionally, VAE and PNEU detect two separate events. If a patient meets a PNEU definition, while this establishes a 14-day RIT for subsequent PNEU events, the PNEU RIT does not apply to or account for the identification of VAEs.
Excluded ventilator modes
In some cases, patients may be on HFV or ECLS or paracorporeal membrane oxygenation for a portion of a calendar day, but not for the entire calendar day, for example when the support is first initiated or when discontinued. In these instances, the patient is eligible for inclusion in VAE surveillance during the portion of the calendar day when the patient was being mechanically ventilated using a conventional mode of mechanical ventilation (not on HFV or ECLS or paracorporeal membrane oxygenation). You should review the FiO2 and PEEP data recorded for the portion of the calendar day when the patient was NOT on HFV or ECLS or paracorporeal membrane oxygenation to select the daily minimum FiO2 and PEEP.
Once the patient has been switched to HFV or ECLS or paracorporeal membrane oxygenation they are no longer included in VAE surveillance. On calendar days when the patient was on HFV or ECLS or paracorporeal membrane oxygenation for the entire day (specifically, midnight to 11:59 pm), you will not record a daily minimum FiO2 or PEEP – you will enter “Not applicable” or “Not eligible for surveillance” in your worksheet column and you will not enter values in the VAE calculator for daily minimum FiO2 and PEEP for that particular day.
Once the patient has been switched back to a conventional mode of mechanical ventilation, VAE surveillance may resume. If the patient has been on HFV or ECLS or paracorporeal membrane oxygenation for one or more calendar days (such that there is a gap in recording of the daily minimum FiO2 and PEEP), then upon return to a conventional mode of mechanical ventilation you will essentially need to start over with VAE surveillance. The patient would need to have at least 2 days of stability or improvement and at least 2 days of worsening oxygenation on the ventilator identified before you can detect a VAE.
For example, if the patient was on conventional mechanical ventilation on January 10 until 10:00 am, switched to HFV at 10:00 am, remained on HFV until 1:00 pm on January 11 and was then placed back on a conventional mode of mechanical ventilation, you would be able to evaluate the FiO2 and PEEP values recorded for the patient from midnight to 10:00 am on January 10 (period on conventional mechanical ventilation) and from 1:00 pm to 11:59 pm on January 11 (period on conventional mechanical ventilation) when looking for VAEs. In contrast, if a patient was on HFV for the entire calendar day on January 10 and January 11, then you would exclude them from VAE surveillance during this period. Once the patient returns to conventional mechanical ventilation for some portion of each calendar day you could again include in VAE surveillance and once again begin daily assessment for the daily minimum FiO2 and PEEP values obtained while the patient was on the conventional mode of ventilation.
The exclusion for ECLS and paracorporeal membrane oxygenation is related to the oxygenation of the blood. When ECLS or paracorporeal membrane oxygenation is in place, the blood is artificially oxygenated via a membrane oxygenator. The use of the oxygenator does not allow for correct application of the VAC portion of the VAE protocol (identifying evidence of respiratory deterioration based on PEEP and FiO2 settings); therefore, the exclusion is related to meeting the VAC criteria.
If VAC criteria are met prior to initiation of ECLS or paracorporeal membrane oxygenation, then a VAE is identified and the patient would not be excluded from being evaluated for IVAC or PVAP. The VAE should be reported at the highest level of the algorithm met.
In the example below, the patient meets VAC criteria on MV day 5, the onset date of worsening oxygenation. ECMO is initiated on MV day 7. The patient also has a fever of 39.2°C on MV day 7 and has 4 QADs on MV days 5-8. Initiation of ECMO before IVAC criteria are fulfilled would not impact assessment of the patient for IVAC (or PVAP) since VAC criteria are met prior to ECMO initiation. IVAC criteria are met in this example, and the VAE would be reported as an IVAC.
| MV Day | 3 | 4 | 5 | 6 | 7 | 8 |
| VAC Criterion | Baseline Day 1 | Baseline Day 2 | Worsening Day 1 | Worsening Day 2 | ||
| IVAC Criteria | QAD | QAD | QAD
Fever 39.2°C |
QAD | ||
| Vent Mode | ACV | ACV | ACV | ACV | ECMO | ECMO |
Weaning/mechanical ventilation liberation trials and VAE
Yes. As long as the patient is receiving support from a mechanical ventilator and is eligible for VAE surveillance, then you should review all FiO2 and PEEP data that are recorded each day to identify the daily minimum FiO2 and PEEP values – including FiO2 and PEEP values that are recorded during periods of time when the patient is undergoing spontaneous awakening or spontaneous breathing trials (or other forms of weaning from mechanical ventilation). The only periods of time that are not taken into consideration when identifying the daily minimum FiO2 and PEEP values are times when the patient is on HFV, ECLS, or paracorporeal membrane oxygenation, or times when the patient is not receiving mechanical ventilation support (for example, a T-piece trial or a trach collar trial where the patient continues to receive supplemental oxygen but is receiving no additional support from the mechanical ventilator). Keep in mind, too, that during periods of time when the patient is being mechanically ventilated using APRV or a related strategy (see the APRV FAQ), you will only review FiO2 data (not PEEP).
Daily minimum values
Definitions of “daily minimum PEEP” and “daily minimum FiO2” can be found in the VAE protocol. Please refer to the VAE protocol for details. There will be multiple PEEP and FiO2 settings documented each calendar day on mechanically ventilated patients. These PEEP and FiO2 values are typically recorded in the paper or electronic medical record, on respiratory therapy and/or nursing flow sheets, in the section of the flow sheet that pertains to respiratory status/mechanical ventilation. You will make daily minimum value determinations using documented settings specific to the calendar day and independently of the settings recorded on the previous calendar day or the next calendar day. Please note that the VAE surveillance protocol specifies to use the daily minimum PEEP and FiO2 values when assessing for both the period of stability or improvement and the period that indicates worsening oxygenation.
From the multiple PEEP and FiO2 settings that will be documented each calendar day, you will identify the minimum (lowest) value for that calendar day that is maintained for > 1 hour.
- If PEEP/FiO2 settings are documented less frequently than every hour throughout the calendar day (for example, every 2 hours or every 4 hours), each setting will have been maintained for > 1 hour so the daily minimum PEEP/FiO2 will be the lowest value documented on the calendar day.
- If PEEP or FiO2 settings are documented hourly or more frequently you will need multiple consecutive recordings of that PEEP or FiO2 setting.
-
- If PEEP/FiO2 settings are monitored and recorded every 15 minutes, you would need 5 consecutive recordings of a particular PEEP/FiO2 setting for that setting to be identified as the daily minimum value (for example, at 09:00, 09:15, 09:30, 09:45, and 10:00).
-
- If PEEP/FiO2 settings are monitored and recorded every 30 minutes, you would need 3 consecutive recordings (for example, at 09:00, 09:30, and 10:00).
-
- If PEEP/FiO2 settings are monitored and recorded hourly, you would need 2 consecutive recordings (for example, at 09:00 and 10:00).
- If there is no PEEP/FiO2 setting that was maintained for > 1 hour, then select the lowest setting documented for that calendar day.
Consider the following examples:
Example # 1 (Mechanical ventilator data from a single day, May 10):
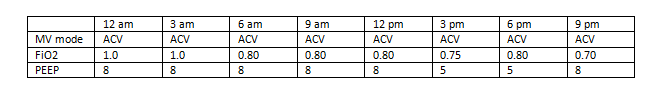
In this example, the daily minimum FiO2 for May 10 would be recorded as 0.70 (70%), and the daily minimum PEEP would be recorded as 5 cmH2O. Note that the daily minimum FiO2 may have been documented at a different time than the daily minimum PEEP (as in the example above).
Example # 2 (Mechanical ventilator data from a single day, May 11):
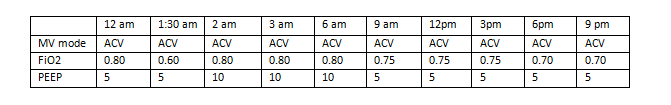
In this example, the daily minimum FiO2 for May 11 would be recorded as 0.70 (70%), and the daily minimum PEEP would be recorded as 5 cmH2O. Note that even though the lowest recorded FiO2 value for the day was 0.60 (60%), it was only recorded at a single time point with an interval indicating that it was not maintained for > 1 hour.
Selecting the daily minimum value on a calendar day is not indicating the patient was stable at that one value (of PEEP or FiO2) throughout the day – it is saying that value was the lowest setting the patient was able to tolerate for greater than 1 hour during that calendar day. Operationally you will always be collecting/recording/evaluating those values, at the earliest, on the following day so that you can allow for the values obtained for the full 24-hour calendar day to be assessed.
When assessing for VAC, you are not comparing values that occur within a calendar day to determine stability, improvement, or worsening; you are looking for stability, improvement, or worsening in the daily minimum values across calendar days. You will compare the daily minimum value from day to day within the individual parameters (PEEP and FiO2), looking for a period of stabilization or improvement in PEEP followed by a period of worsening oxygenation in PEEP, or a period of stabilization or improvement in FiO2 followed by a period of worsening oxygenation in FiO2.
PEEP values between 0 cmH2O and 5 cmH2O
For the purposes of VAE surveillance, PEEP values between 0 cmH2O and 5 cmH2O are considered equivalent. This means that patients with a daily minimum PEEP in the range of 0-5 cmH2O must have an increase in the daily minimum PEEP to at least 8 cmH2O, sustained at or above 8 cmH2O for at least 2 calendar days, in order for the VAC definition to be met. In essence, think of values between 0-5 as all being equal to 5, and therefore an increase to 8 cmH2O is necessary to satisfy the required increase in daily minimum PEEP ≥ 3 cmH2O over the daily minimum PEEP in the baseline period.
NOTE: The VAE calculator will automatically make this correction. PEEP values entered into the VAE calculator that are between 0-5 cmH2O will be interpreted by the calculator as equal to 5.
APRV
You would only disregard PEEP values on calendar days when the patient was mechanically ventilated using APRV or a related mode of mechanical ventilation for the entire calendar day (specifically, from midnight through 11:59 pm). On calendar days when the patient was on APRV for the entire day, you will not record a daily minimum PEEP – you will enter “Not applicable” in your worksheet column for daily minimum PEEP for that particular day. Likewise, when using the online VAE Calculator, do not enter a daily minimum PEEP value on days when the patient was on APRV for the entire calendar day. Leave the PEEP field in the VAE Calculator empty/blank for these days (DO NOT ENTER ZERO).
While patients are mechanically ventilated using APRV or a related mode they are included in VAE surveillance, but when assessing these patients for VAE, you will use only FiO2 data to identify periods of stability or improvement and worsening. In some cases, patients may be mechanically ventilated using APRV or a related mode for a portion of a calendar day, but not for the entire calendar day. In these instances, you should look at all FiO2 data recorded for the entire calendar day when selecting the daily minimum FiO2, and you should look at the portion of the calendar day when the patient was NOT on APRV or a related mechanical ventilation mode to select the daily minimum PEEP. In other words, when recording the daily minimum PEEP for a patient who spent part of the day on APRV and part of the day on a conventional mode of mechanical ventilation, you will review PEEP values just from the portion of the day when the patient was on a conventional mode of mechanical ventilation.
For example, on January 1 a patient is switched from conventional mechanical ventilation at 11:00 am to APRV. The patient remains on APRV until January 2 at 11:00 pm, when the patient is switched back to conventional mechanical ventilation. You will review the FiO2 data from the entire day on January 1 and January 2, and the PEEP data that were recorded for the period from midnight to 10:59 am on January 1 (since the patient was on conventional mechanical ventilation during this time) and from 11:00 pm to 11:59 pm on January 2 (since the patient was back on conventional mechanical ventilation at this time). You will be able to assign a daily minimum PEEP for each of these days based on the time spent on conventional mechanical ventilation, and a daily minimum FiO2 based on each entire calendar day, and review both PEEP and FiO2 data to determine whether there is a VAE.
Here is another example: On January 1 a patient is switched from conventional mechanical ventilation at 11:00 am to APRV. The patient remains on APRV all day on January 2, and on January 3 until 11:00 pm, when the patient is switched back to conventional mechanical ventilation. In this example, you will (as above) have PEEP data to review for January 1 and for January 3, based on the amount of time the patient was on conventional mechanical ventilation. But because the patient was on APRV all day on January 2, you will need to rely on the FiO2 to determine whether there is a VAE during that period of days. (Because there is a gap in PEEP data, you’ll have to start over looking for a baseline period in PEEP on January 3).
Temperature and WBC count
Yes. As long as there is an abnormal temperature (> 38°C or < 36°C) or abnormal white blood cell count (≥ 12,000 cells/mm3 or ≤ 4,000 cells/mm3) documented during the VAE Window Period, it should be used in determining whether the patient meets the IVAC definition, regardless of whether an abnormal temperature or abnormal white blood cell count was also present on admission or outside the VAE window period.
Antimicrobial agent Appendix
The antimicrobial criterion is one of the required criteria in the Infection-related Ventilator-Associated Complication (IVAC) definition. The IVAC definition was not originally developed to identify respiratory infections alone; therefore, the list of antimicrobial agents eligible for meeting the IVAC antimicrobial criterion was broad and included drugs that are not used to treat respiratory infections. This caused concern and confusion among some users – particularly in situations where the “new” antimicrobial agent that resulted in an IVAC determination and then subsequently a PVAP determination was not an agent used to treat a respiratory infection.
To avoid increasing the complexity of this already complex criterion, the list was refined and selected antimicrobial agents were removed: oral cephalosporins and penicillins, erythromycin and erythromycin/sulfisoxazole, amantadine, rimantadine, chloramphenicol, tinidazole, fidaxomicin, nitrofurantoin, enteral vancomycin, and daptomycin. These are agents that would not be used, or would be unlikely to be used, in treating a lower respiratory infection in a critically ill patient.
Antimicrobial agent
No. With the IVAC definition, the intent is not to specifically identify infectious events arising from the respiratory tract – it is to identify an event that may be infectious in nature (whether arising from the lungs or elsewhere) that is associated with a period of respiratory deterioration. Any antimicrobial agent that is found in the antimicrobial agent appendix and is administered within the correct timeframe and for the required period of time stated in the protocol will be used to satisfy the antimicrobial criteria for IVAC. Therefore, when determining if a patient satisfies the IVAC definition, there is no need to discern the reason for the administration of the antimicrobial.
QADs
In a patient who has met the VAC definition and has additionally met the temperature and/or WBC requirement for IVAC but dies prior to meeting the requirement for ≥ 4 Qualifying Antimicrobial Days, the IVAC criteria are not fulfilled. In this instance a VAC (not an IVAC) would be reported to NHSN.
Culture results
For purposes of the VAE surveillance protocol, qualitative refers to identification of an organism or cells without a quantity descriptor: for example, “Staphylococcus aureus present” or “white blood cells seen.” Semi-quantitative refers to a text description of the amount or quantity of organism or cells present, without a specific numeric value: for example, “occasional,” “few,” “moderate,” “many,” “heavy” or 1+, 2+, 3+, 4+. An example of semi-quantitative reporting would be a result indicating “many Pseudomonas aeruginosa” or “1+ epithelial cells.” Quantitative refers to a specific numeric description of the amount of organism or cells present: for example, “105 CFU/ml Klebsiella pneumoniae.”
Yes, it does not matter if the patient had previous positive cultures for certain organisms – if an eligible pathogen is recovered from an eligible specimen with a collection date during the VAE window period, it should be used in determining if PVAP is met.
Excluded pathogens
This means the following are excluded:
- All Candida species – those that have been identified to the species level such as Candida albicans, those that are reported as Candida species, and also to include culture reports that may simply say, for example, “many yeast isolated”
- All coagulase-negative Staphylococcus species – for example, coagulase-negative Staphylococci, Staphylococcus epidermidis, Staphylococcus species
- All Enterococcus species – for example, Enterococcus species, Enterococcus faecalis, VRE
Specimen
Yes. For the purposes of VAE surveillance, a “bronchial wash” is considered the same type of specimen as a bronchoalveolar lavage (BAL).
Specimens may frequently be labeled as “sputum” when they are really “endotracheal aspirates.” Making the automatic substitution is not advised. If, however, you can verify with the patient’s caregiver that the specimen was indeed an endotracheal aspirate, and also confirm that your microbiology laboratory does not process specimens labeled as “sputum” differently than those labeled as “endotracheal aspirate,” the culture result can be used to meet the requirements for the PVAP definition. Additionally, take the opportunity to address improving specimen labeling.
Cytology findings
Yes, lower respiratory tract specimen cytology findings in support of identification of infection can be used to satisfy PVAP Criterion 3.
Secondary BSI to lower respiratory events in locations performing VAE surveillance
For purposes of NHSN, for a bloodstream infection to be deemed secondary to a site-specific infection (specifically, related to an infection at another site, such that primary site of infection may have seeded the bloodstream secondarily), the patient must first meet one of the NHSN site-specific definitions. For example, for a bloodstream infection to be deemed secondary to PNEU, the PNU2 or PNU3 definition must be met first. You cannot attribute a bloodstream infection secondary to PNEU based on a clinical diagnosis of pneumonia or solely based on recovery of a matching pathogen from a lower respiratory tract specimen and blood specimen.
To determine if a positive blood culture can be attributed as a secondary bloodstream infection (BSI) related to a lower respiratory tract event, consider the following steps:
1) Does the patient meet any of the VAE definitions?
- If only the VAC or IVAC definition is met, then the positive blood culture CANNOT be secondary to the VAE (as per the VAE surveillance protocol, BSIs cannot be deemed secondary to VAC or to IVAC). EXAMPLE: A patient hospitalized and mechanically ventilated in the MICU for 14 days develops worsening oxygenation following a 7-day period of stability on the ventilator. VAC criteria are met on hospital day 15 (stable ventilator settings on days 12 and 13, increased ventilator settings on days 14 and 15). The date of event is hospital day 14. The white blood cell count is noted to be 15,500 cells/mm3 on hospital day 14. Meropenem and intravenous vancomycin are started on hospital day 15, administered through the patient’s right-sided central line, which was inserted on ICU admission. The antibiotics continue to be administered on hospital day 18, meeting IVAC criteria. Endotracheal aspirate cultures done on hospital days 15 and 16 grow scant upper respiratory flora. A blood culture collected on hospital day 15 is positive for Klebsiella oxytoca. There are no other signs or symptoms of infection. This patient should be reported as having an IVAC and a central line-associated BSI if the BSI cannot be attributed as secondary to another primary site of infection. The BSI cannot be reported as secondary to the IVAC event.
- If the PVAP definition is met, then the bloodstream infection may be attributed to the VAE (as a secondary BSI) IF the blood culture meets the VAE secondary BSI requirements as outlined in the VAE surveillance protocol: the organism isolated from blood must match an organism isolated from the respiratory tract culture used in meeting the PVAP definition AND the blood culture must be collected during the 14-day VAE event period.
EXAMPLE: Patient hospitalized and mechanically ventilated in the MICU for 14 days develops worsening oxygenation following a 7-day period of stability on the ventilator. VAC criteria are met on hospital day 15 (stable ventilator settings on days 12 and 13, period of worsening oxygenation on hospital days 14 and 15). The date of event is hospital day 14. The white blood cell count is noted to be 15,500 cells/mm3 on hospital day 14. Meropenem and vancomycin are started on hospital day 15, administered through the patient’s right-sided central line (inserted on ICU admission). The antibiotics continue to be administered on hospital day 18, meeting IVAC criteria. Endotracheal aspirate specimens collected on hospital days 15 and 16 grow ≥ 105 CFU/ml Klebsiella oxytoca. A blood culture collected on hospital day 15 is positive for K. oxytoca. This patient should be reported as having a PVAP with a secondary BSI due to K. oxytoca.
2) If the BSI cannot be attributed as a secondary BSI to VAE, then the positive blood culture can be evaluated to see if it is secondary to any of the infection sites as defined in Chapter 17 or PNEU, UTI, or SSI event protocols. If another specific site infection to which the bloodstream infection can be attributed as a secondary BSI is not identified, the BSI may need to be reported as a primary BSI/CLABSI.
Secondary BSI pathogens
When a bloodstream infection is deemed secondary to a PVAP (specifically, BSI diagnosed by blood culture collected during the 14-day VAE event period, with at least one organism from blood matching an organism isolated from an eligible respiratory tract specimen obtained during the VAE window period), organisms isolated from that same blood culture that do not match an organism in the eligible respiratory tract specimen MAY be reported as a PVAP pathogen—EXCEPT when they are one of the excluded organisms (specifically, Candida species or yeast not otherwise specified [NOS], Enterococcus species, coagulase-negative Staphylococcus species). An exception to the excluded organism rule is made when the eligible respiratory tract specimen is pleural fluid or lung tissue. Excluded organisms isolated from positive blood cultures must be accounted for as a secondary bloodstream infection to another site-specific infection) or as a primary bloodstream infection. Please see the examples 1-4 below.
NOTE: When multiple, separate blood cultures are positive during the 14-day VAE event period, ONLY those blood cultures that are positive for at least one organism matching an organism isolated from an eligible respiratory tract specimen obtained during the VAE window period may be considered secondary to the PVAP.
Example 1
A PVAP was detected in a patient in the MICU, with an event date of August 1. The PVAP determination was made based on an endotracheal aspirate culture collected on August 2 (within the VAE window period) that was positive for many Pseudomonas aeruginosa (PA). On August 9, within the 14-day event period, the patient has a positive blood culture for PA and Escherichia coli (EC). This positive blood culture should be reported as a secondary BSI for the PVAP event because it occurred within the 14-day event period, and because at least one of the pathogens isolated from blood matches an organism isolated from the respiratory tract specimen. Pathogens reported for this PVAP should include PA and EC.
Example 2
A PVAP was detected in a patient in the MICU with an event date of August 1. The PVAP determination was made based on an endotracheal aspirate culture collected on August 2 (within the VAE window period) that was positive for ≥ 105 CFU/ml Pseudomonas aeruginosa (PA) and Candida albicans (CA). On August 9, within the 14-day event period, the patient has a positive blood culture for PA and CA. This positive blood culture should be reported as a secondary BSI for the PVAP event because it occurred within the 14-day event period, and because at least one of the pathogens isolated from blood matches an organism isolated from the respiratory tract specimen. Pathogens reported for this PVAP should be limited to PA. Candida albicans CANNOT be reported as a pathogen for PVAP because it is an excluded pathogen when isolated from endotracheal aspirate. CA must be accounted for as either a secondary bloodstream infection to another primary site-specific infection or as a primary bloodstream infection.
Example 3
A PVAP was detected in a patient in the MICU with an event date of August 1. The PVAP determination was made based on a lung biopsy obtained for culture on August 2 (within the VAE window period) that was positive for Pseudomonas aeruginosa (PA) and Candida albicans (CA). On August 9, within the 14-day event period, the patient has a positive blood culture for PA and CA. This positive blood culture should be reported as a secondary BSI for the PVAP event because it occurred within the 14-day event period, and because at least one of the pathogens isolated from blood matches an organism isolated from the respiratory tract specimen. Pathogens reported for this PVAP should include PA and CA. In this instance the Candida albicans CAN be reported as a pathogen for this VAE because it is isolated from a culture of lung tissue.
Example 4
A PVAP was detected in a patient in the MICU with an event date of August 1. The PVAP determination was made based on a lung biopsy obtained for culture on August 2 (within the VAE window period) that was positive for Pseudomonas aeruginosa (PA). On August 9, within the 14-day event period, the patient has a positive blood culture for PA and Candida albicans (CA). This positive blood culture should be reported as a secondary BSI for the PVAP event because it occurred within the 14-day event period, and because at least one of the pathogens isolated from blood matches an organism isolated from the respiratory tract specimen. Pathogens reported for this PVAP should be limited to PA. Candida albicans CANNOT be reported as a pathogen for this VAE because it is an excluded pathogen unless isolated from pleural fluid or lung tissue. The lung tissue culture in this case did NOT grow CA. As an excluded organism isolated from positive blood culture, CA must be accounted for as either a secondary bloodstream infection to another primary site-specific infection or as a primary bloodstream infection.
Brain injury/ brain death in ventilated patients
Brain injury and/or brain death alone is not sufficient for exclusion from VAE surveillance. The requirement is that if the VAE date of event (date of onset of worsening oxygenation) is on or after the date of documentation in the medical record of evidence of consent for organ donation AND the patient is being supported for organ donation purposes, then the event should not be reported as a VAE. If the requirement is not met, the patient is not excluded from surveillance, and a VAE should be reported at the highest level met in the VAE algorithm if you are conducting in-plan VAE surveillance for the location.
VAE upgrades
Per the VAE surveillance protocol, only one VAE can be reported during each 14-day event period (where day 1 is the onset of worsening oxygenation). A previously detected VAE cannot be “upgraded” using information obtained outside of the original VAE window period. Once the VAC definition is met the other criteria needed to satisfy the IVAC and PVAP definitions must all be present within the VAE window period timeframe, according to the protocol. The temperature, white blood cell count, and laboratory test collection dates must occur within the VAE Window Period, and the antimicrobial agent(s) used to satisfy the ≥ 4 qualifying antimicrobial days (QADs) criterion must be “new” within the VAE window period. Keep in mind that while the antimicrobial agent must be new within the VAE window period, QADs that count toward satisfying the IVAC antimicrobial criterion may occur outside the VAE Window Period.
Example: A VAC is detected in a medical ICU patient, with the day of onset of worsening oxygenation occurring on mechanical ventilation (MV) day 10. The VAE window period is therefore determined to be from MV day 8 (2 days before the onset of worsening oxygenation) through MV day 12 (2 days after the onset of worsening oxygenation). The patient has a temperature of 39°C on MV day 10 and is started on a new antimicrobial agent on MV day 11 (with that new agent continued for 7 consecutive days, from MV day 11 through MV days 17). The IVAC definition is therefore met. On MV day 15, a BAL is performed and it grows 105 CFU/ml Pseudomonas aeruginosa. Because the BAL specimen was collected OUTSIDE of the VAE window period (even though it was collected during the 14-day event period), it cannot be used to upgrade the VAE from an IVAC to a PVAP.
Episode of mechanical ventilation
Per the VAE surveillance protocol, the 14-day event period is to be observed even if a new episode of mechanical ventilation (MV) is established during that event period. The 14-day event period for VAE surveillance is governed by the date of event (date of onset of worsening oxygenation), not the date of initiation of mechanical ventilation. So, if a patient is removed from mechanical ventilator for one full calendar day or more and then is returned to the ventilator within the 14-day event period, a new VAE cannot be detected or reported until the 14 days have elapsed. When the patient is returned to the ventilator, a new episode of MV would begin, and the mechanical ventilation day count would start over again. The earliest a new VAE could be identified would be day 3 of the new MV episode.
In the example presented in the table below, you will see that there is a VAC detected on hospital day 4 during the first episode of mechanical ventilation. The patient is extubated on hospital day 6 and remains off MV for one full calendar day (hospital day 7). On hospital day 8, the patient is re-intubated, thereby starting a second episode of MV. The patient is observed to meet VAC criteria, with a baseline period of stability or improvement on hospitals days 8 and 9 and a period of worsening on hospitals days 10 and 11 – but because the patient is still within the 14-day event period for the VAE detected on hospital day 4, a new VAE is not reported.
| Hosp Day No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| My Episode | 1 | 1 | 1 | 1 | 1 | 1 | —- | 2 | 2 | 2 | 2 | 2 |
| MV Day No. | 1 Intubated at noon |
2 | 3 | 4 | 5 | 6 Extubated at noon |
—- | 1 Re-Intubated at 0800 |
2 | 3 | 4 | 5 |
| VAE Criterion | — | Baseline Day 1 | Baseline Day 2 | Worsening Day 1 | Worsening Day 2 | —- | Baseline Day 1 | Baseline Day 2 | Worsening Day 1 | Worsening Day 2 | ||
| VAE | VAC | No VAC | ||||||||||
| 14-day Event Period | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
Ventilator data
See Scenarios A through E below.
Scenario A:
Patient is intubated by the EMS in the field or is intubated in the ED. FiO2 and PEEP data are available from the time the patient spent in the ED, prior to the patient being transferred to the ICU as an inpatient. Should I use the pre-hospital/ED ventilator data when making VAE determinations for that patient?
No. Ventilator data that is obtained from patients in the Emergency Department or other pre-hospital/pre-inpatient locations should not be included in VAE surveillance. Therefore, VAE surveillance begins for patients who are intubated in the pre-hospital or ED setting upon transfer to an inpatient location. Day 1 of ventilator data consists of data collected during the first calendar day of inpatient care.
Scenario B:
Patient is intubated and mechanically ventilated in an inpatient unit where VAE surveillance is not occurring. The patient is transferred to another inpatient unit in the same hospital where VAE surveillance is occurring. Do I use ventilator data from the transferring unit, even though VAE surveillance was not occurring in that unit?
Yes – to an extent. Since the transferring unit is in the same hospital, and since ventilator data from that transferring unit should be readily available, we advise that you go back 2 calendar days prior to transfer and utilize minimum daily PEEP and FiO2 data from the transferring unit to determine whether a VAE has occurred during the first 2 days in the receiving unit. If a VAE is detected with an onset date on calendar day 1 or 2 (day of or day after transfer) in the receiving unit, that VAE would be attributable to the transferring unit and so would not be reported (since the transferring unit was not doing VAE surveillance).
Scenario C:
Patient is intubated and mechanically ventilated in an inpatient unit where VAE surveillance is occurring. The patient is transferred to another inpatient unit in the same hospital where VAE surveillance is also occurring. Do I use ventilator data from the transferring unit?
Yes. When transferring a patient between units that are both participating in VAE surveillance, surveillance should continue in an ongoing fashion. For example, if the patient had a VAE in the transferring unit on August 1, and was transferred to the receiving unit on August 4, a new VAE could not be detected in the receiving unit until the 14-day event period for the August 1 VAE had elapsed (so, August 15 in this case).
Scenario D:
Patient is intubated and mechanically ventilated in another hospital or healthcare facility and then transferred to my facility. It is unknown whether the transferring facility was performing VAE surveillance or not. Should I use ventilator data from the transferring facility (if available in my facility’s medical record) when making my VAE determinations?
When ventilator data are available from a transferring facility and documented in your facility’s medical record, you may use the ventilator data from the 2 calendar days prior to transfer to determine whether a VAE has occurred early in the course of the inpatient stay in your receiving facility. As in Scenario B, above, if a VAE is detected with onset date on calendar day 1 or 2 (day of or day after transfer) in your receiving facility, the VAE would be attributable to the transferring facility. If no ventilator data are available from the transferring facility, VAE surveillance begins on admission to your receiving facility/inpatient location where VAE surveillance is taking place.
Scenario E:
Patient is intubated and mechanically ventilated in another hospital or healthcare facility and then transferred to my facility. The transferring facility was performing VAE surveillance, and I have been informed that a VAE was detected in the transferring facility five days prior to transfer. Upon arrival in my receiving facility, does the 14-day event period apply, or do I need to “start fresh” with ventilator data available in my facility?
You should “start fresh,” although as noted above in Scenario D, you can use ventilator data from the 2 calendar days prior to transfer to determine whether there is a VAE early in the course of hospitalization in your receiving facility that would be attributed back to the transferring facility.
Home ventilators
The first step in determining whether such patients should be included in VAE surveillance is to decide whether the patient is on invasive mechanical ventilation, as defined by the NHSN. The NHSN definition of a ventilator is, “Any device used to support, assist or control respiration (inclusive of the weaning period) through the application of positive pressure to the airway when delivered via an artificial airway, specifically an oral/nasal endotracheal or tracheostomy tube.” Note: Ventilation and lung expansion devices that deliver positive pressure to the airway (for example, CPAP, BiPAP, Bi-level, IPPB, and PEEP) via non-invasive means (for example, nasal prongs, nasal mask, full face mask, total mask, etc.) are not considered ventilators unless positive pressure is delivered via an artificial airway (oral/nasal endotracheal or tracheostomy tube).
Based on this definition, patients on home mechanical ventilators or patients supported by devices typically considered non-invasive ventilator devices should be included in VAE surveillance if the ventilator support is administered via an endotracheal or tracheostomy tube, even if the support is administered only for portions of each day (for example, overnight). Patients receiving non-invasive ventilation (such as BiPAP via a face mask or nasal mask) should not be included in VAE surveillance.
The second step in determining whether such patients can be included in VAE surveillance is to determine whether the FiO2 or PEEP can be set at a specific level on the home mechanical ventilator or other ventilator device. Our current understanding is that some brands of home mechanical ventilators and devices typically used for non-invasive ventilation do not have the capability of setting a specific FiO2 or PEEP level. In these circumstances, a patient could not be included in VAE surveillance because it would not be feasible to assess changes in the set level of FiO2 or PEEP. If the FiO2 or PEEP can be set at a specific value and changes in the settings can be monitored, then these patients should be included in VAE surveillance.
If the patient is switched from a home mechanical ventilator or other device to a critical care unit mechanical ventilator, then they can be included in VAE surveillance at that time (taking into account that a baseline period of stability or improvement will need to be established on the critical care mechanical ventilator).
NOTE: Patients on home ventilators in locations where in-plan VAE surveillance is being performed will be included in the ventilator day denominator count regardless of their eligibility for VAE surveillance.
Location of mechanical ventilation
This field should reflect the location where the patient was intubated. For example, if the patient was intubated by first responder personnel in the field prior to arrival in the facility where mechanical ventilation was eventually initiated, the location chosen should be Mobile Emergency Services/EMS. If the patient was intubated at another facility, the location chosen should be “Location outside facility.” You will want to map these locations into your facility’s Locations list as appropriate.
Date of mechanical ventilation initiation
When a patient is admitted to a facility on a mechanical ventilator the date of mechanical ventilation initiation should reflect the actual date of mechanical ventilation initiation, not the date of admission to the facility. If necessary, an estimate of the actual date of mechanical ventilation initiation can be used. In the situation where a patient’s mechanical ventilation is initiated prior to admission to a facility, only in circumstances where the actual date or an estimate of the actual date cannot be determined should the date of mechanical ventilation initiation default to the date of admission to the facility.
VAE specific events
It is important to note that having an IVAC or PVAP is not necessarily “worse” than having a VAC – the algorithm is progressive in terms of criteria to be met (from VAC to IVAC to PVAP), but this is not to imply that each subsequent tier is more clinically significant than the one before. The fundamental definition within the algorithm is the VAC definition (which is defined on the basis of respiratory deterioration) – so even in those circumstances where an IVAC or PVAP is detected, the event still met the VAC definition. It’s just that there is additional evidence that the event may be infectious in nature (IVAC) as opposed to non-infectious, and if infectious in nature the infection may be related to the lower respiratory tract (PVAP).
Benchmarking
The rates and SIRs that are potentially appropriate for these purposes include the overall VAE rate or Total VAE (where the numerator includes all events meeting at least the VAC definition – VAC + IVAC + PVAP). You may find rates and SIRs for “IVAC-plus” (where the numerator includes all events meeting at least the IVAC definition – IVAC + PVAP) and the rates of the individual specific event types (VAC only, IVAC only, and PVAP only) useful for internal quality improvement purposes. More information regarding the SIR models and parameter estimates for Total VAE and IVAC-plus can be found in The NHSN Guide to the SIR. [PDF – 50 Pages]