Community-Acquired Pneumonia (CAP): Clinical Testing Algorithm for Histoplasmosis
This clinical testing algorithm was made in collaboration with the Mycoses Study Group Education & Research Consortium.
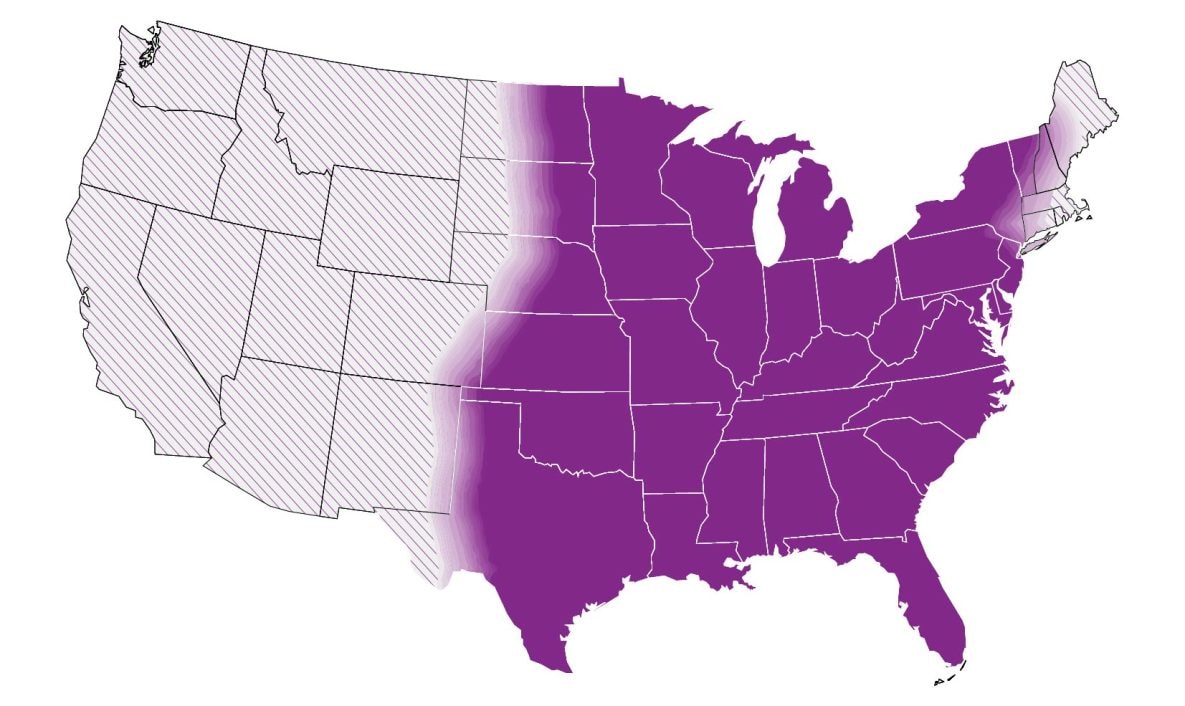
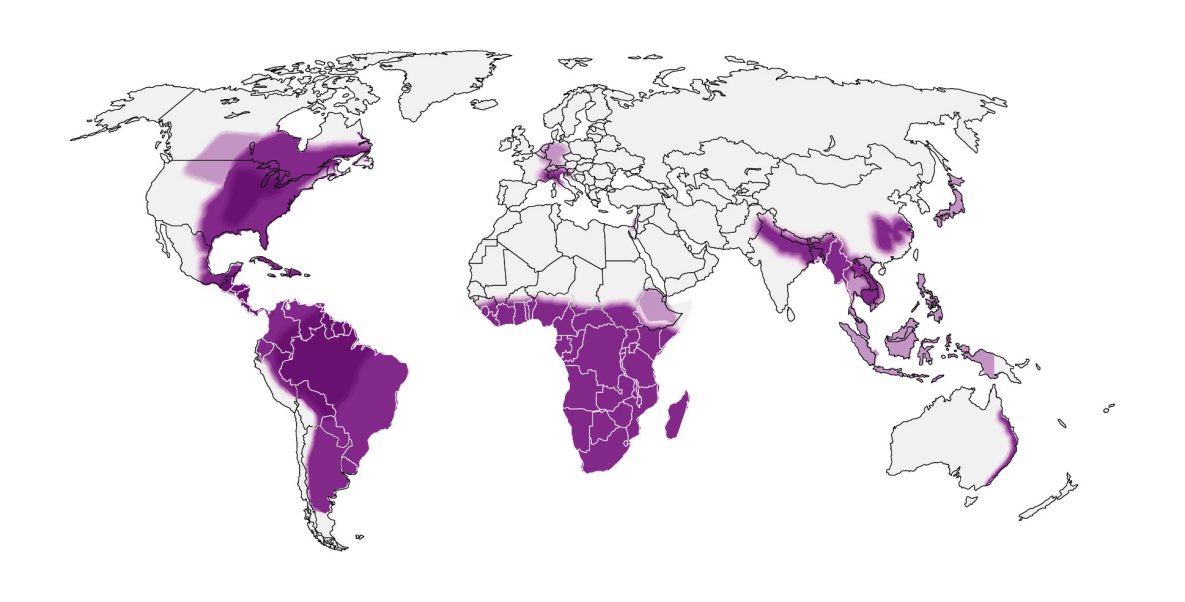
In the United States, histoplasmosis is endemic to the Midwest, South and Northeast. Globally, it is endemic in parts of South and Central America, Africa, Asia and Europe. These maps are approximations. Histoplasma is not distributed evenly and may not be present everywhere within the shaded areas. It may also be present outside of the areas indicated.

If a patient lives in or has traveled to a disease-endemic area and has:
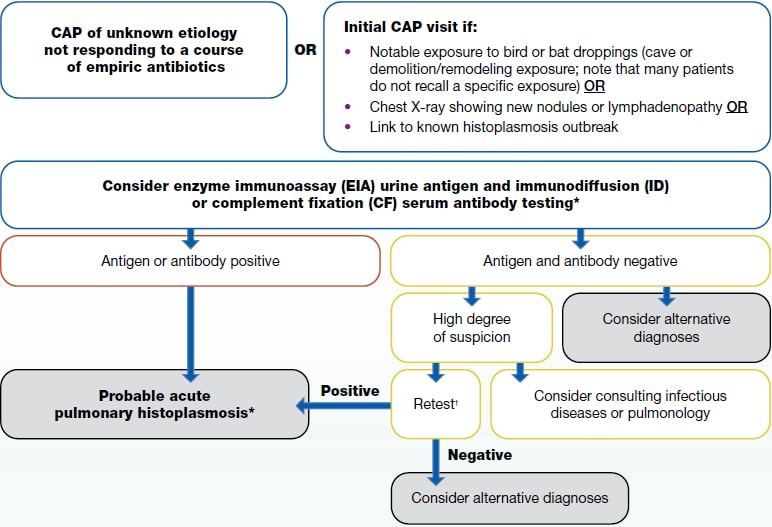
1a. CAP of unknown etiology not responding to a course of empiric antibiotics
or
1b. Initial CAP visit if:
- Notable exposure to bird or bat droppings (cave or demolition/remodeling exposure; note that many patients do not recall a specific exposure) OR
- Chest X-ray showing new nodules or lymphadenopathy OR
- Link to known histoplasmosis outbreak
2. Consider enzyme immunoassay (EIA) urine antigen and immunodiffusion (ID) or complement fixation (CF) serum antibody testing
- Antigen or antibody positive
- Probable acute pulmonary histoplasmosis
- Antigen and antibody negative
- Consider alternative diagnosis
- High degree of suspicion
- Consider consulting infectious disease or pulmonology
- Retest
- Repeat antibody testing, since testing may be negative early in illness, or order sputum or bronchoalveolar lavage (BAL) culture and microscopy.
- Retest is negative
- Consider alternative diagnosis
- Retest is positive
- Probable acute pulmonary histoplasmosis
- Note: in the first two weeks of infection, false-negative tests may occur with antigen testing. Depending on availability, serum antibody testing for Histoplasma can be considered to increase sensitivity, particularly if clinical suspicion is high; however, a positive serum antibody test may indicate previous infection. Enzyme immunoassay (EIA or ELISA) antigen testing is typically considered first because of a quicker turnaround and higher sensitivity; however, it has a high rate of cross-reactivity with Blastomyces. Immunodiffusion and complement fixation antibody tests can be used if EIA is not available or if clinicians want to rule out blastomycosis or other fungal diseases.
Information About Histoplasmosis
See Clinical Infectious Diseases publication, Clinician Outreach and Communications Activity Call, and Medscape Article covering the histoplasmosis algorithm.
- Clinical Testing Guidance for Coccidioidomycosis, Histoplasmosis, and Blastomycosis in Patients With Community-Acquired Pneumonia for Primary and Urgent Care Providers | Clinical Infectious Diseases | Oxford Academic (oup.com)
- Webinar Thursday, September 21, 2023 – Algorithms for Diagnosing the Endemic Mycoses Blastomycosis, Coccidioidomycosis, and Histoplasmosis (cdc.gov)
- Three Cases of Community-Acquired Pneumonia (medscape.com)
About Histoplasmosis
Histoplasmosis is an invasive fungal infection that often presents as community-acquired pneumonia in primary and urgent care settings. Disease can occur in both immunocompetent and immunocompromised persons. Histoplasmosis cannot be reliably distinguished from other causes of respiratory illness by signs or symptoms alone. Thus, patients are commonly misdiagnosed; in some instances, over half of patients may receive inappropriate antibacterial drugs.1 Travel-associated disease is common, and climate change may be influencing the geographical range of Histoplasma; thus, cases can be found in all regions across the United States.2,3
When to Consider Testing for Histoplasmosis
Clinicians may consider testing for histoplasmosis in patients with community-acquired pneumonia who have not improved with at least one course of empiric antibiotics and who live in or have traveled to an area where histoplasmosis is thought to be endemic (central and eastern United States, predominantly in areas around the Ohio and Mississippi River Valleys).
Clinicians may also consider testing for histoplasmosis on an initial presentation of community-acquired pneumonia when patients have any of the following features:
How to Test for Histoplasmosis
We recommend ordering enzyme immunoassay (EIA) urine antigen tests. Clinicians may consider obtaining a concurrent immunodiffusion (ID) or complement fixation (CF) antibody test to increase sensitivity; false positives from previous infection can occur, but ID antibody positivity typically wanes within three years after infection.5 If these tests are negative, clinicians should consider alternative diagnoses. If a high degree of suspicion remains despite negative initial testing, a clinician may consider ordering (or repeating) antibody testing since tests may be negative early in illness. At this point, clinicians might also consider ordering a sputum or bronchoalveolar lavage (BAL) culture and microscopy. Consultation with specialists in infectious diseases or pulmonology may be considered when a high degree of suspicion remains as well.
Histoplasma antigen and antibody tests have extensive cross-reactivity with Blastomyces. Both infections are treated the same way in most forms.
Clinicians should refer to the Infectious Diseases Society of America’s histoplasmosis treatment guidelines or an infectious disease physician when determining therapy after a positive result.6
| Test | Sensitivity | Specificity | Population Studied |
|---|---|---|---|
| Antibody Tests | |||
| EIA antibody8 | 98% | 97% (high cross-reactivity with Blastomyces) | Immunocompromised & healthy populations |
| Complement fixation (CF) antibody9 | 72%–95% | 70%–80% | Adult populations |
| Immunodiffusion (ID) antibody9 | 70%–95% | 100% | Adult populations |
| Antigen tests | |||
| EIA Urine antigen7 | 79% | 99% | Adult population, people living with HIV |
| EIA Serum antigen7 | 82% | 97% | Adult population, people living with HIV |
| Other tests | |||
| Culture10 | 15%–85% | 100% | Acute or subacute, disseminated disease |
| Microscopy/histopathology10 | 9%–43% | 100% | Acute or subacute, disseminated disease |
- Benedict K, McCracken S, Signs K, et al. Enhanced surveillance for histoplasmosis—9 states, 2018–2019. Open Forum Infect Dis. 2020;7(9):ofaa343. doi:10.1093/ofid/ofaa343
- Benedict K, Derado G, Mody RK. Histoplasmosis-associated hospitalizations in the United States, 2001–2012. Open Forum Infect Dis. 2016;3(1):ofv219. doi:10.1093/ofid/ofv219
- Maiga AW, Deppen S, Scaffidi BK, et al. Mapping Histoplasma capsulatum exposure, United States. Emerg Infect Dis. 2018;24(10):1835-1839. doi:10.3201/eid2410.180032
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20(1):115-132. doi:10.1128/CMR.00027-06
- Richer SM, Smedema ML, Durkin MM, et al. Improved diagnosis of acute pulmonary histoplasmosis by combining antigen and antibody detection. Clin Infect Dis. 2016;62(7):896-902. doi:10.1093/cid/ciw007
- Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45(7):807-825. doi:10.1086/521259
- Fandiño-Devia E, Rodríguez-Echeverri C, Cardona-Arias J, Gonzalez A. Antigen Detection in the diagnosis of histoplasmosis: a meta-analysis of diagnostic performance. Mycopathologia. 2016;181(3-4):197-205. doi:10.1007/s11046-015-9965-3
- Abdallah W, Myint T, LaRue R, et al. Diagnosis of histoplasmosis using the MVista Histoplasma galactomannan antigen qualitative lateral flow–based immunoassay: a multicenter study. Open Forum Infect Dis. 2021;8(9):ofab454. doi:10.1093/ofid/ofab454
- Scheel CM, Gómez BL. Diagnostic methods for histoplasmosis: focus on endemic countries with variable infrastructure levels. Curr Trop Med Rep. Published online April 8, 2014. doi:10.1007/s40475-014-0020-0
- Guimarães AJ, Nosanchuk JD, Zancopé-Oliveira RM. Diagnosis of histoplasmosis. Braz J Microbiol. 2006;37(1):1-13. doi:10.1590/S1517-83822006000100001