About
CDC simulated clade I mpox outbreaks from close-contact transmission within and between U.S. households. Results indicate this transmission route is unlikely to result in a large number of mpox clade I cases in the U.S.
November 21, 2024
This report is one of two modeling studies exploring the potential spread of clade I mpox in the United States. This study explores the impact of household and other non-sexual contact on the potential spread of clade I mpox in the United States. A separate study explores the impact of population-level immunity and transmissibility on sexual transmission among gay, bisexual, and other men who have sex with men (MSM).
At a glance
There is an ongoing outbreak of clade I mpox in Central and Eastern Africa. As of November 16, there has been one case reported in the United States, with no onward spread. CDC simulated clade I mpox outbreaks resulting from close-contact transmission within and between households (household clusters) in the United States to better understand the potential impact of this transmission route. Modeling results indicate:
- Close-contact transmission within and between households is unlikely to result in a large number of mpox clade I cases in the United States. Household transmission clusters would most likely involve 10 or fewer mpox clade I cases, with minimal spread between households.
- Clade I mpox outbreaks involving children would likely be self-limiting in the United States.
- While we did not consider other routes of transmission, a separate CDC modeling study found that among selected U.S. counties with population-level immunity lower than 50%, increases in immunity decrease the chance of prolonged transmission among gay, bisexual, and other men who have sex with men (MSM).
Background
The outbreak of mpox in DRC is caused by the clade I monkeypox virus, which is distinct from the clade II monkeypox virus that caused the ongoing global outbreak that began in 2022. In previous outbreaks in endemic African countries, clade I has caused a higher proportion of severe disease and has been more transmissible than clade II. As of November 18, 2024, one case of clade I mpox has been reported in the United States; monitoring efforts continue, including wastewater monitoring for community detection and testing a high proportion of presumed mpox specimens with tests that can identify mpox by clade.
The outbreak of clade I mpox in Eastern and Central Africa has likely resulted from transmission through several modes in different settings, including household, zoonotic, and sexual exposures. Approximately 70% of suspected mpox cases in DRC in 2024 were in children under age 15, similar to historical observations. The high number of cases in children under 15 suggests that factors outside of sexual transmission have important roles. In this analysis, we explore the implications of spread within the United States through household and other non-sexual close-contact transmission. Transmission caused by close contact within households has occurred in clade I mpox outbreaks in endemic countries. Household clusters have typically been small, although occasionally have involved up to six generations of transmission.
Here we assess the potential size of outbreaks resulting from transmission within and between households in the United States. U.S. transmission dynamics could differ from the situation in DRC.
Key Findings
Modeling results indicate that close-contact transmission within and between households is unlikely to result in a large number of mpox clade I cases in the United States. Household transmission clusters would most likely involve 10 or fewer mpox clade I cases, with minimal spread between households.
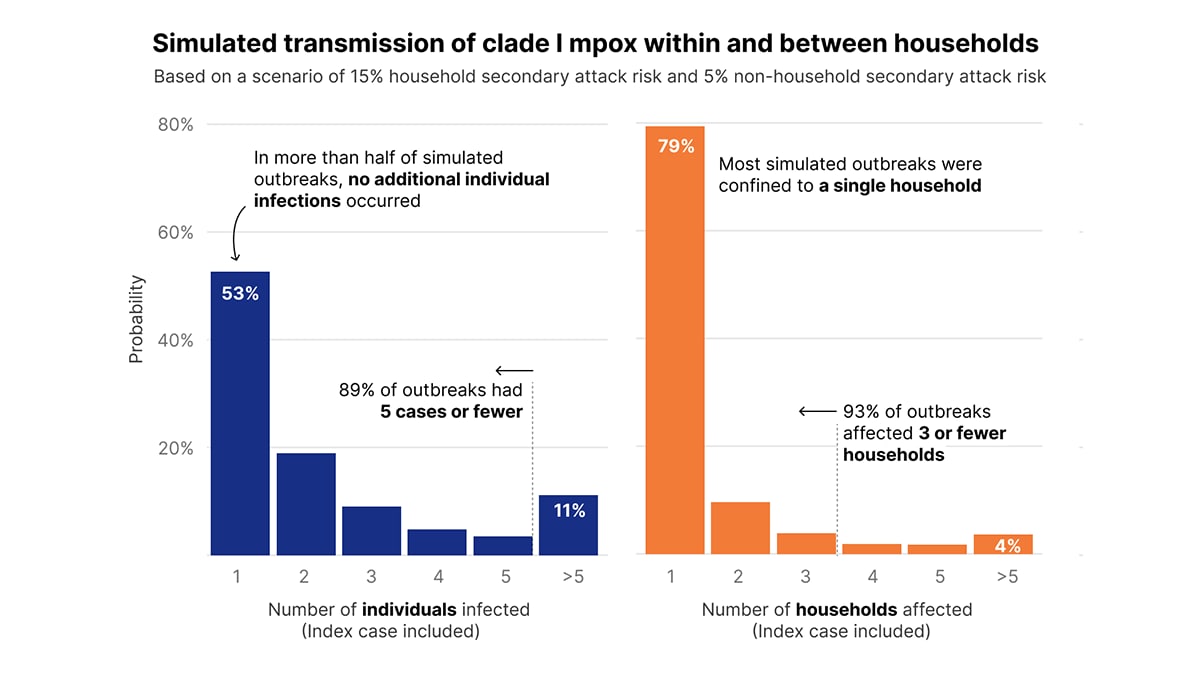
Simulated outbreaks resulted in a minimal number of cases, even considering a plausible “worst-case” scenario based on existing data—with a 15% household secondary attack risk and a 5% non-household (between-household) secondary attack risk. In this scenario, 89% of resulting outbreaks had 5 cases or fewer, and 95% of resulting outbreaks had 10 cases or fewer. In addition, this scenario demonstrated minimal spread between households, with 93% of simulations affecting 3 or fewer households (Figure 1). Due to limitations and caveats of this analysis, these results likely represent an upper bound for transmission driven by non-sexual contact (see Limitations section).
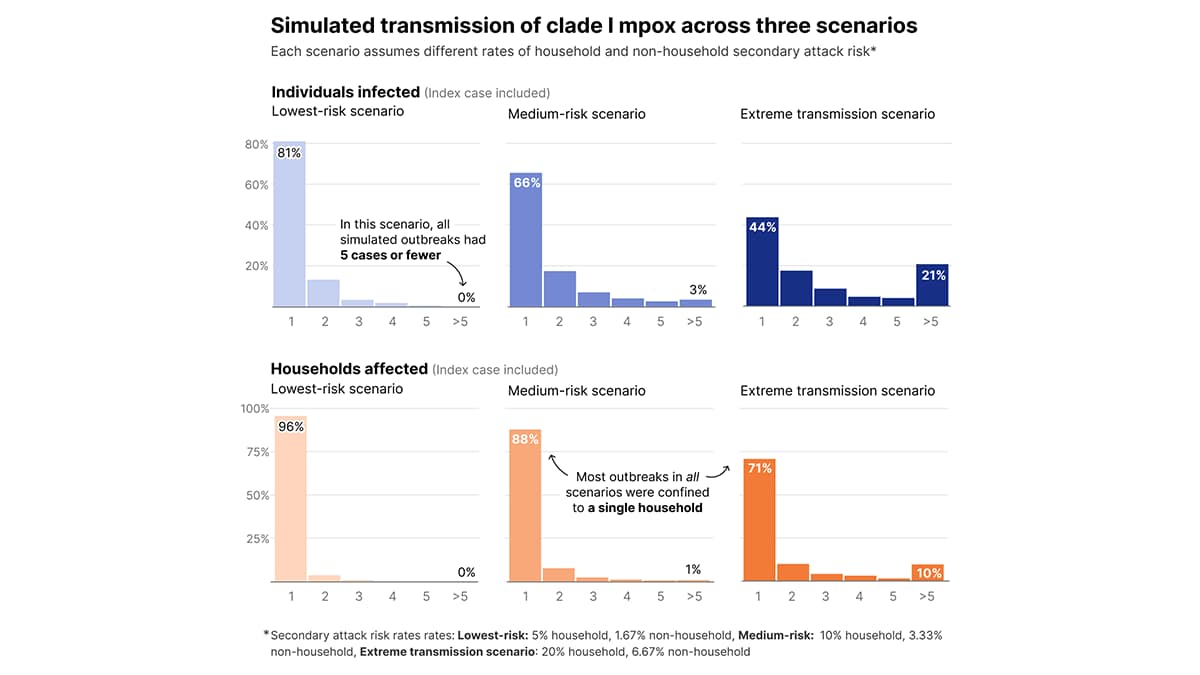
Given the range of household secondary attack risks observed in the literature, we conducted a sensitivity analysis exploring results under three additional transmission scenarios (Figure 2). This analysis assumed the per-contact, non-household transmission risk was one-third the per-contact household transmission risk (see Additional Assumptions section). In the lowest risk scenario, with a household secondary attack risk of 5%, all simulated outbreaks had 5 cases or fewer. Additionally, even in medium-risk and extreme transmission scenarios, with household secondary attack risks of 10% and 20% respectively, the majority of outbreaks in all simulations were confined to a single household.
Public Health Considerations
Although modeling indicates household transmission is unlikely to result in a large number of mpox clade I cases, CDC will continue to evaluate available data and assess the risk posed to the United States by clade I mpox. Learn more about how to protect communities and prevent the spread of mpox.
Methods and Limitations
We developed an agent-based model of clade I mpox outbreak transmission within and between households in the United States. We generated a synthetic population of 5,000 U.S. households with people in three different age groups: children under 5 years old, children 5-17 years old, and adults 18 years old and older, with household size and age composition based on U.S. census data. The mean household size in our simulated population was 2.45 people. In the simulation, all household members interact equally, and any contact between households is due to children interacting with each other in high-contact settings, such as daycares, or through close friendships (see Additional Assumptions section).
We assumed a mean incubation period of 8 days and a mean infectious period of 27 days, with no interventions accounted for in the model. Household and non-household secondary attack risk were parameterized based on existing epidemiological evidence.
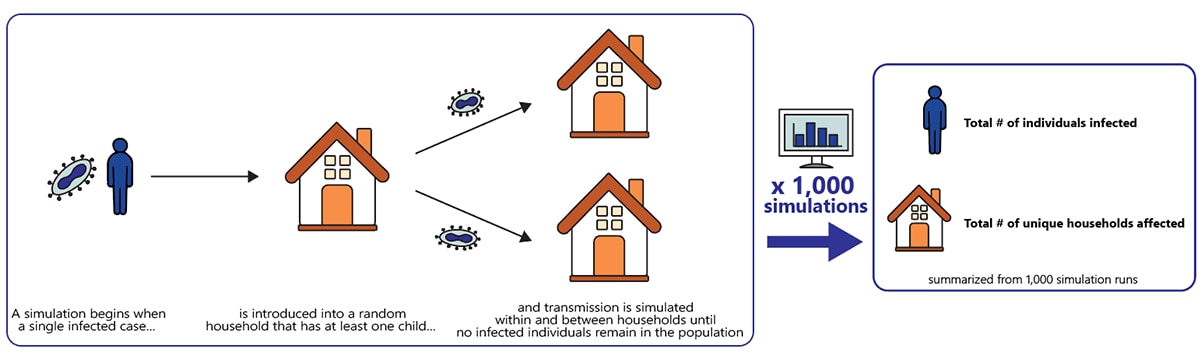
For each outbreak simulation, we introduced a single infected case into a random household that had at least one child. The simulation was run until no infected individuals remained in the population. The total number of individuals who were infected and the total number of unique households affected were summarized from 1,000 simulation runs (Figure 3).
Interactions between contacts
We assumed all household members interact equally and that any contact between households was due to children interacting with each other in high-contact settings, such as daycares, or through close friendships. We simulated non-household contacts using data on group size recommendations in child-care settings for contacts between children under 5 years old, and survey data on the number of close friends for contacts between children over 5 years old.
Transmission
The population modeled was susceptible to infection, with no immunity through vaccination or prior infection. Parameters for within-household transmission and between-household transmission were based on the household secondary attack risk and non-household secondary attack risk data reported from outbreaks in the DRC (Beer and Rao), as this data is not available from the United States. The within-household transmission rate varied from 5% to 15%, while the between-household transmission rate varied from 0%-4.8% in unvaccinated contacts (Fine et al., Jezek et al., and McMullen et al.). This analysis assumed the per-contact, between-household transmission risk was one-third the per-contact, household transmission risk based on estimates from Beer and Rao.
Our model does not account for transmission from infected adults to those outside their household, whether through sexual or other types of contact. In our model, only children could infect children in a different household, and we did not assume transmission between children and adults in other settings, such as daycares. Therefore, our results could underestimate outbreak size. On the other hand, the model assumes that the initial case always occurs in a household with at least one child, which could increase outbreak size, since it increases the average size of households initially infected and raises the likelihood of between-household transmission. The simulated population was assumed completely susceptible with no immunity through vaccination or prior infection. The model also did not incorporate interventions or mitigation measures, such as patient isolation, that may reduce transmission risk. As such, these results most likely provide an upper bound for transmission scenarios driven by non-sexual contact.
