About
To assess the impact among MSM of potential clade I monkeypox introduction into the U.S., CDC modeled varying levels of transmissibility and county-specific immunity. Modeling indicated that counties with higher population-level immunity had lower chances of a prolonged or large outbreak.
November 26, 2024
This report is one of two modeling studies exploring the potential spread of clade I monkeypox in the United States. This study explores the impacts of population-level immunity and transmissibility on sexual transmission of monkeypox among gay, bisexual, and other men who have sex with men (MSM) and is an update of the original technical report, which evaluated 13 of the 50 counties analyzed here. A separate study explores the impact of household and other non-sexual spread on number of cases and number of households infected in an outbreak.
At a Glance
Clade I monkeypox is causing an ongoing outbreak in the Democratic Republic of the Congo (DRC) and neighboring countries in Central and Eastern Africa. In some places, this outbreak has spread predominantly through intimate or sexual contact between adults. During the ongoing clade II monkeypox outbreak that began in 2022, the main transmission route was associated with sexual activity among certain gay, bisexual, and other men who have sex with men (MSM). CDC modeled potential clade I monkeypox transmission among MSM to explore potential impacts of introduction to these sexual networks in the United States. The model accounted for varying levels of transmissibility and county-specific population-level immunity from previous infection, estimated undiagnosed infections, and receipt of one or two doses of the JYNNEOS vaccine. Our modeling results indicate that:
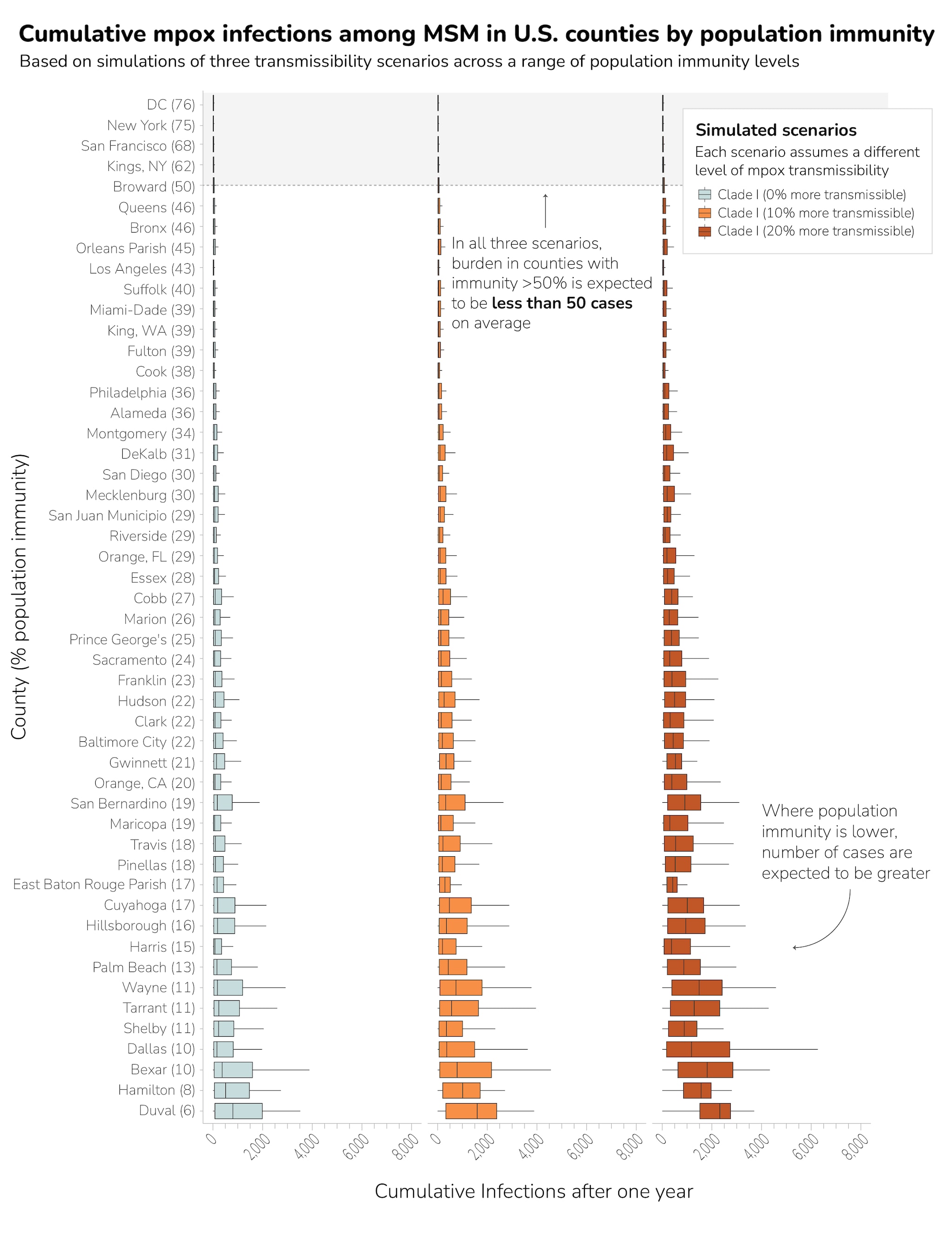
- In 23 out of the 50 U.S. counties included in the analysis, simulated clade I outbreaks among MSM were smaller than ongoing clade II outbreaks across three transmissibility scenarios. The clade I outbreaks were likely smaller because of prior immunity from vaccination and previous infection, as well as the population's related behavior change.
- The size and duration of potential monkeypox outbreaks varied across individual counties due to differences in population-level immunity, defined as a combination of estimated undiagnosed infections, vaccination coverage and previous clade II monkeypox case rates.
- Counties with higher population-level immunity had lower chances of a prolonged or large outbreak. Therefore, CDC recommends all eligible people get vaccinated.
Background
In previous outbreaks in endemic African countries, clade I virus has caused a higher proportion of severe disease and been more transmissible than clade II within close-contact settings. As of November 16, 2024, one case of clade I monkeypox has been detected in the United States.
The current DRC outbreak of clade I monkeypox has likely resulted from transmission through several modes in different settings, including household, zoonotic, and sexual exposures. A 2024 outbreak in the Kamituga mining region in DRC has been associated with transactional sex, as 88% of hospitalized cases reported recent transactional sex. Additionally, health authorities reported six cases among MSM in a rural town in DRC in 2023. In a risk assessment updated as of November 18, 2024, CDC assessed the risk posed by the clade I monkeypox outbreak in the DRC to the U.S. MSM population as low to moderate— higher than the risk posed to the general population.
Here, we use a dynamic, agent-based transmission model to explore the chance of prolonged sexual transmission and potential outbreak sizes among MSM in 50 counties in the United States if clade I monkeypox virus were introduced to this population. The 50 counties selected for this analysis are those included in the Ending the HIV Epidemic (EHE) Initiative and represent a range of population size and immunity profiles (see Methods for additional information). Given uncertainty around the transmissibility of clade I monkeypox, we explored three levels of clade I transmissibility: equally transmissible as clade II (74.5% per-contact transmissibility), 10% increased transmissibility over clade II, and 20% increased transmissibility over clade II.
Key findings
The county-level modeling results indicated the size and duration of potential monkeypox outbreaks varied across individual counties due to differences in population-level immunity. Results indicated that outbreaks averaging more than 50 cases did not occur when population-level immunity from vaccination or previous infection was greater than 50% among MSM (Figure 1). Additionally, our simulation results showed that counties with higher population-level immunity had smaller outbreaks (defined as cumulative infections one year after introduction) and lower chance of prolonged transmission (defined as continued incident infections one year after introduction) (Figure 2). In the baseline scenario assuming clade I is as equally transmissible as clade II, no county with population-level immunity above 21% had outbreaks larger than their clade II outbreaks (Appendix Table 1).
Our results suggest that high population-level immunity in a county, coupled with related behavior change, would lead to a much smaller monkeypox clade I outbreak among MSM compared to the ongoing monkeypox clade IIb outbreak. When comparing modeling results to the number of confirmed diagnoses in the 2022 outbreak, only nine of the 50 counties had higher median numbers of monkeypox model-diagnosed cases across all transmissibility scenarios modeled. However, 27 of 50 counties had higher median monkeypox diagnoses in at least the 20% more transmissible scenario. All of these counties had estimated levels of population-level immunity below 35%.
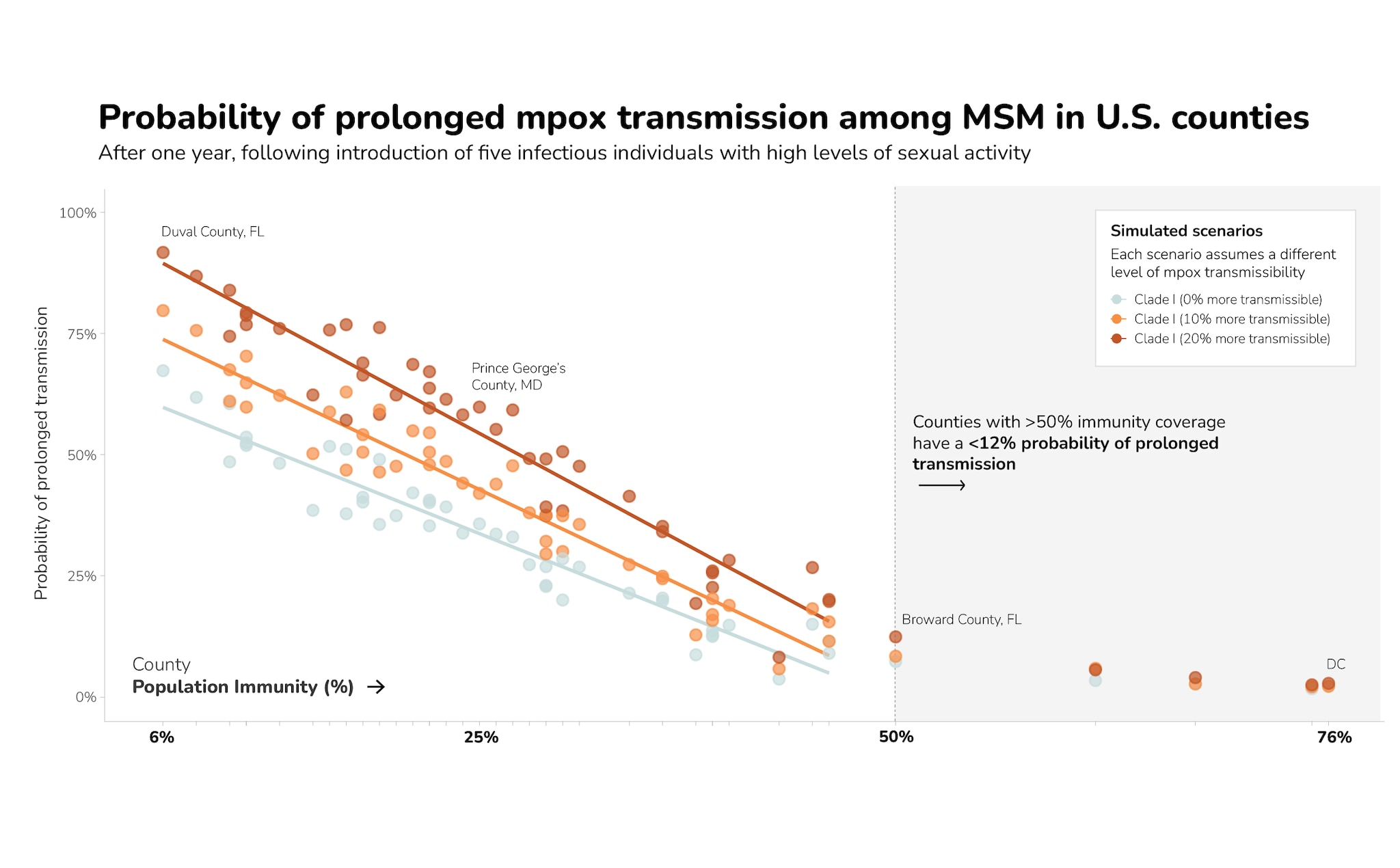
Among counties with population-level immunity under 50%, higher levels of immunity were correlated with lower probabilities of prolonged transmission among MSM for all three levels of transmissibility we analyzed (Figure 2). These results suggest that for counties with higher levels of population-level immunity, the probability of prolonged transmission is lower. In particular, counties with >50% population-level immunity had a <12% probability of prolonged transmission.
Public Health Considerations
Increasing population-level immunity through vaccination in counties can reduce both the number of infections as well as the chance of prolonged transmission of monkeypox. Vaccination is an important—yet underutilized—tool in preventing the spread of monkeypox. The Advisory Committee on Immunization Practices recommends people with potential risk of exposure to monkeypox receive two doses of the JYNNEOS vaccine. Despite this, only 25% of the approximately two million people recommended to receive the vaccine in the United States based on risk of exposure to clade II monkeypox have received both doses, and an additional 15% of those eligible have received one of the two recommended doses. JYNNEOS is also expected to protect against clade I monkeypox. CDC will continue to evaluate available data and assess the risk posed to MSM in the United States by the clade I monkeypox outbreak in DRC.
If you are a public health partner interested in learning more about your county-specific population-level immunity, please contact us.
Methods
We developed an agent-based model to simulate monkeypox sexual transmission among MSM. We adapted a previous model that assessed clade II transmission in MSM networks, adding new data on U.S. sexual network structures and exploring transmission parameters to represent clade I monkeypox.
We produced simulations for all 50 counties in the Ending the HIV Epidemic (EHE) Initiative. The 50 EHE jurisdictions account for more than half of all new HIV diagnoses, and many represent urban areas across the United States with large MSM populations. For each of the 50 counties, we established baseline population-level immunity based on quarterly monkeypox vaccination data as of August 2024, estimates of undiagnosed infections, and monkeypox clade II case reporting as of October 1, 2024, from DCIPHER, a cloud-based CDC data platform. We assumed that prior infection with clade II monkeypox provides full protection against both monkeypox clades, and that vaccination with the JYNNEOS vaccine provides partial but strong immunity (75.2% and 85.9% for one dose and two doses, respectively).
We then generated sexual networks with sizes equivalent to the estimated MSM population for each county as a set of three interconnected dynamic networks representing main (long-term), casual (shorter-term), and one-time sexual partnerships among MSM. The sexual behavior within the networks was estimated from an online survey of MSM from across the United States. We assumed that the distribution of type and frequency of sexual behavior (e.g., the proportion of people who form long-term, short-term, and/or one-time partnerships) is the same across all counties as data does not exist to estimate these parameters at a local level. While these patterns of sexual behavior remain constant between counties, each modeled county is unique in terms of population size and initial immunity conditions. Finally, we also included short-term behavioral adaptations in our modeling as a reduction in the frequency of spontaneous or one-time sexual encounters. We assumed a level of behavioral adaptation in our analyses that was similar to what occurred in the District of Columbia in 2022, which was based on previous modeling work and which was documented across the United States.
The cumulative number of infections and proportion of simulation runs with infections remaining one year after introduction of the virus were compared across three transmission scenarios. Transmission scenarios included the following: 1) baseline, clade I equally transmissible as clade II (74.5%); 2) clade I 10% more transmissible than clade II (81.9%); and 3) clade I 20% more transmissible than clade II (89.4%). For each simulation, we assumed that five MSM with the highest levels of sexual activity—defined as having one or more spontaneous/one-time sexual partners per week in addition to any main or casual partners—were exposed to monkeypox and ran the simulation for one year. We summarized results across 1,000 simulation runs for each county and scenario combination, both as a median and interquartile range (IQR) of cumulative diagnosed monkeypox infections after one year following introduction (Appendix Table 2) and as the probability of prolonged monkeypox transmission (Appendix Table 3).
The updated model was fit to sexual network data that was collected more recently (2017-2019), representing MSM across the United States rather than a single geographic region. Furthermore, we added data on oral sex partnerships in addition to anal sex partnerships and recalibrated sexual activity group strata to better characterize the range of sexual activity reported in the data. We also calibrated the clade II transmissibility parameter using clade II monkeypox case data from early in the 2022 outbreak. This parameter has a calibrated distribution of β (4.24, 1.45), with mean equal to 74.5% probability of transmission per contact.
We also added additional transmission parameters to explore possible clade I scenarios. While there are no studies that estimate the exact difference in per-contact transmissibility between the globally circulating clade II virus and clade I in humans, there is evidence that rash intensity and detectable viral loads are greater for clade I relative to clade II in traditional zoonotic and household transmission settings, and a small-mammal model demonstrated that virulence of clade I is greater than clade II. We generalized these lines of evidence, assuming a 10% increase relative to clade II (81.9% per-contact transmissibility) and a 20% increase (89.4% per-contact transmissibility).
Lastly, previous work modeled a range of immunity to monkeypox using a single population size, where in this work we modeled county-specific MSM population size and composition of population-level immunity. This generates more variance in our results, but both approaches come to similar conclusions about the overall level of population-level immunity that is protective against prolonged transmission of monkeypox.
Population-level immunity was calculated as the proportion of each county's population with increased risk of monkeypox exposure that had some form of protective immunity, defined as either a previous monkeypox virus infection (diagnosed or undiagnosed), one dose of vaccine, or two doses, and weighted by the amount of protection each type of immunity provides the individual against future infection, as assumed in the model. We used JYNNEOS vaccine administration data through August 2024, diagnosed monkeypox cases through October 1, 2024, and an estimate of the number of undiagnosed monkeypox infections based on previous modeling work. We estimated the size of the MSM population with increased risk of monkeypox exposure in each county using county-level estimates from survey data reduced by 40% to reflect the smaller proportion of MSM considered to have higher sexual activity based on national survey data. There are 11 EHE counties not included in the above set of county-level estimates. For these counties, we instead estimated the size of the population with increased risk of monkeypox exposure following previous work, as the summation of the total number of MSM living with HIV and the number of PrEP-eligible MSM, plus 10%.
Limitations
Our analysis is subject to several limitations, including some that could lead to underestimation of outbreak size and probability of prolonged transmission. We assumed that prior infection with clade II monkeypox provides full immunity against both monkeypox clades (no development of monkeypox following exposure), that vaccination with the JYNNEOS vaccine provides partial immunity (significantly reduced probability of monkeypox development following exposure), but did not account for waning immunity from either previous infection or vaccination in this model. We also assumed that the JYNNEOS vaccine and prior infection with clade II will provide similar levels of immunity against clade I as for clade II, all of which could reduce our estimates of outbreak size. In addition, variations in vaccination reporting requirements between counties—including some that have stopped reporting to CDC since immunity estimates were previously published and/or have individual opt-in reporting—may mean that true population immunity could be higher than is estimated here and outbreak sizes could be smaller than modeled. Lastly, the number of infections reported in these counties and included in our analysis may be lower than the actual number of infections, given under-detection and reporting of cases.
We also made several assumptions that could have led to overestimates of outbreak size and probability of prolonged transmission. We seeded simulations with a high number of infections in highly connected individuals. In addition, we assumed substantial behavioral adaptation in the face of an outbreak. Our sensitivity analysis for clade II indicated that substantially larger outbreaks could occur if this assumption is violated, though outbreak sizes did not exceed 100 cases on average for counties with >50% immunity among MSM with increased risk of monkeypox exposure (defined in our model as MSM who are likely to form spontaneous or one-time partnerships in addition to having main and/or casual partners). We also assumed that no vaccination occurred during the simulated year. In counties where analysis indicates that large outbreak sizes are possible, we expect—but did not model—that additional health interventions would be implemented that could potentially reduce outbreak sizes. This analysis only explicitly modeled 50 counties; therefore, it is not representative of the entire U.S.
Finally, we acknowledge substantial uncertainty in the size of the MSM population with increased risk of monkeypox exposure (which affects coverage estimates) and note that sexual networks may not be accurately reflected for all counties, as the survey data could not be disaggregated to the county level. While unaccounted-for geographic variation in sexual behavior could influence the absolute outbreak size in a given county, because previous modeling work used networks with lower levels of sexual activity and had similar outcomes, we expect that the general conclusions presented here would hold.
Appendix
Table 2: Median (IQR) Cumulative Monkeypox Diagnoses in First 365 Days, all 50 EHE Counties
Table 3: Probability of Prolonged Monkeypox Transmission within all 50 EHE Counties (After one year, following introduction of five infectious individuals with high levels of sexual activity)
Previous Updates
This technical brief was updated on November 26, 2024. The brief posted on July 4, 2024 can be found here.
