Considerations for Selecting Protective Clothing used in Healthcare for Protection against Microorganisms in Blood and Body Fluids
Background
Healthcare workers can be exposed to biological fluids that are capable of transmitting diseases. Those diseases, which are caused by a variety of microorganisms such as, Hepatitis B virus (HBV), Hepatitis C virus (HCV), Ebola Virus, and Human Immunodeficiency Virus (HIV) can pose significant risks to life and health. Healthcare workers wear protective clothing (e.g., surgical gowns, isolation gowns, and coveralls) to protect both patients and themselves from the transfer of microorganisms by blood and body fluids. A common misunderstanding among many end users is that they are protected from blood, body fluids, and other potentially infectious materials when they wear any type of fluid-resistant garment. This document provides an overview of scientific evidence and information on national and international standards, test methods, and specifications for fluid-resistant and impermeable gowns and coveralls used in healthcare. This document focuses on selecting protective clothing primarily on the basis of their barrier properties; it does not address all aspects of garments related to their design, integrity, durability, comfort, and functionality.
Classifying Worker Exposure to Bloodborne Pathogens
As with any type of personal protective equipment (PPE), the key to proper selection and use of gowns and coveralls is to understand the hazards and the risk of exposure. The Centers for Disease Control and Prevention (CDC) has categorized three primary routes of transmission: (i) contact (direct and indirect), (ii) respiratory droplets, and (iii) airborne droplet nuclei [Siegel 2007]. Contact transmission is generally the most common and direct contact occurs when microorganisms transfer directly from one person to another. Airborne transmission occurs by dissemination of either airborne droplet nuclei or small particles in the respirable size range containing infectious agents. Droplet transmission refers to respiratory droplets generated through coughing, sneezing, or talking. By using appropriate protective clothing, it is possible to create a barrier to eliminate or reduce contact and droplet exposure, and therefore prevent the transfer of microorganisms between patients and healthcare workers. This document provides information about protective clothing standard test methods and classification standards when the transmission of the microorganisms is through direct contact with blood or body fluids. Direct contact can occur through broken skin or mucous membranes located areas such as the eyes, nose, or mouth. In addition to blood, other body fluids can include (but are not limited to) urine, saliva, sweat, feces, vomit, breast milk, and semen.
Employers should conduct a thorough risk assessment first to identify potential exposures to blood and body fluids. The risk of exposure sometimes depends on the stage of the disease and severity of symptoms. For example, for Ebola virus disease, severe symptoms are strongly associated with high levels of virus production. In addition, close contact with the patient and invasive medical care can increase opportunities for transmission. This should be considered during the risk assessment, such as in the case of Ebola virus disease, as Ebola patients can release large volumes (as much as 8 liters/day) of body fluids (vomit, diarrhea) [Kreuels 2014]. A complete assessment of the risks is outside the scope of this document, but resources are available. For example, the Association for the Advancement of Medical Instrumentation (AAMI) published a guidance document on selection and use of protective apparel in healthcare facilities, Technical Information Report (TIR) 11 [AAMI 2005]. Some of the factors important to assessing the risk of exposure in health facilities include source, modes of transmission, pressures and types of contact, and duration and type of tasks.
Selecting Protective Clothing
Once the hazard and the risks of exposure are identified, gown and coverall selection can be guided by current scientific understanding of how protective clothing materials provide protection against microorganisms in blood and body fluids. A microorganism’s movement through protective clothing materials depends upon several factors, including the following:
- Physical and chemical properties of the fabric: Includes factors such as thickness pore size, and repellency
- Shape, size, and other characteristics of the microorganisms: Includes factors such as morphology, motility, and adaptation to environmental extremes
- Characteristics of the carriers: Includes factors such as surface tension, volume, and viscosity
- External factors: Includes factors such as physical, chemical, and thermal stresses
Several different microorganisms have been found in healthcare settings, including bacteria, viruses, and some fungi. The shape and size of microorganisms varies, and this will affect their ability to move through a fabric structure. In general, fungi are larger than bacteria, and bacteria are larger than viruses. For instance, HIV virus is spherical and 100–120 nanometers (nm) in diameter. The Ebola virus is a single-stranded RNA virus with a filamentous shape, a median particle length ranging from 974 nm to 1,086 nm, and average 80 nm in diameter.
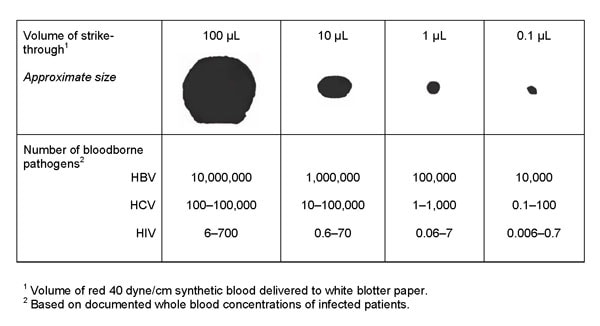
Microorganisms are transported by carriers such as body fluids, sloughed skin cells, lint, dust, and respiratory droplets. A significant number of microorganisms can be carried in a very minute volume of blood or body fluids, which may not be visible to the naked eye (see Figure 1). For example, the number of infectious units of Hepatitis B in a 0.1-microliter (µL) droplet is 10,000, which is why it is highly infectious and easily transferrable. Ebola virus RNA levels in blood also increase rapidly during the acute phase of the illness. One study reported an average peak titer of 3.4 x 105 RNA copies per 0.1 µL (i.e., 34 times higher than the concentration of Hepatitis B) for cases associated with a fatal outcome [Towner 2004]. Several studies [Brown 1992; Kotilainen et al. 1992; Shadduck et al. 1990; McCullough 1993] have also reported that when liquid containing microorganisms penetrate a material, microorganisms are carried with it, and penetration is possible without liquid being visible. Because of this, standardized test methods must be sensitive enough to detect microorganism penetration, since this is the only way to determine if microorganism penetration has occurred in any part of the garment, including the seams.
Figure 1: Bloodborne pathogen strikethrough (penetration) conversion chart (This chart converts the amount of strikethrough to the amount of potential bloodborne pathogen contamination). The four spots at the top were formed from premeasured droplets of synthetic blood and marked in microliters (µL) ranging from 100 µL to 0.1 µL. Adapted with permission from AAMI TIR 11:2005, “Selection and use of protective apparel and surgical drapes in health care facilities.”
One challenge that employers such as hospitals and pre-hospital emergency care organizations face is selecting the most appropriate protective clothing for healthcare workers based on the recommendations, practices, and regulations. This challenge is complex because there are several terms (e.g., fluid-resistant, fluid-proof, impermeable, and impervious) used in the industry to define barrier resistance properties of garments. The term “fluid-resistant” usually refers to fabrics that resist liquid penetration, but may allow penetration with pressure. According to Taber’s Cyclopedic Medical Dictionary, “impermeable” means not allowing passage, as of fluids; impenetrable [Thomas 1997]. Calling a fabric “impermeable” or “impervious” usually means that the fabric prevents liquids or microorganisms from penetrating. Impermeable could be in reference to water, to blood, to viruses, or to all. The terms “impervious” and “impermeable” are often used interchangeably. Unfortunately, there is no industry consensus for using these terms. Therefore, manufacturers usually provide fabric or garment specifications associated with the standard test methods or standard classifications. In addition, due to the misleading use of these terms, the FDA does not approve marketing surgical gowns or drapes with “impervious,” “impermeable,” “fluid repellant,” or “fluid-resistant” labeling claims.
Evidence-based guidance is needed in order to define these terms to improve communication among employers/purchasers and gown and coverall manufacturers/suppliers. In this document, we use the term “fluid-resistant” to apply to protective clothing tested against water as the liquid challenge and reserve the use of the term “impermeable” to materials that have demonstrated blockage of microorganisms using a recognized standard test method (discussed later).
Employers should consider the garment design as part of their selection process. Unfortunately, no clinical studies have been done to compare the efficacy of gowns vs. coveralls. Both have been used effectively by healthcare workers in clinical settings during patient care. Thus, other factors need to be considered when comparing gowns and coveralls during the selection process. While the material and seam barrier properties are essential for defining protection, the coverage provided by the material used in the garment design, as well as certain features including closures, will greatly affect protection. For example, a coverall with a front zipper closure could result in the compromise of barrier protection if the ordinary cloth and plastic zipper used in its construction is not covered with a flap of barrier material that can be sealed to the garment. Similarly, most of the surgical gowns rated for high levels of barrier protection may include the high-performance barrier materials in only certain portions of the gown (sleeves and front panel). This is especially important when contact from hazardous/contaminated fluids can come from multiple directions.
In general, there is a significant difference between the design of traditional coveralls and isolation/surgical gowns. Although coveralls typically provide 360-degree protection because they are designed to cover the whole body, including back and lower legs and sometimes head and feet as well, the design of surgical/isolation gowns do not provide continuous whole-body protection (e.g., possible openings in the back, coverage to the mid-calf only) (see Figure 2).

Figure 2: Examples of a typical coverall and an isolation gown.
Gowns, on the other hand, are relatively easier to put on and, in particular, to take off. They are generally more familiar to healthcare workers and hence more likely to be used and removed correctly. These factors also facilitate training in their correct use. During patient care, the risk of the anticipated exposure is typically in the area of front chest and sleeves, thus gowns are used frequently in health care. The level of heat stress generated due to the added layer of clothing is also expected to be less for gowns than coveralls due to several reasons, which include the openings in the design of gowns and total area covered by the fabric. For gowns, it is important to have sufficient overlap of the fabric so that it wraps around the body to cover the back (ensuring that if the wearer squats or sits down, the gown still protects the back area of the body).
Employers should consider some of the critical fabric and clothing properties (e.g., strength properties of the fabric and seams [e.g., tensile strength and seam strength], barrier properties of seams/closures, size of the garment, etc.) when selecting the appropriate protective clothing. If the fabric or seams and barrier layer on the fabric is not durable enough to withstand typical stresses applied during wear or use (e.g., if wrong size garment is used), garments may tear during kneeling, reaching, or bending. In addition, garments too large for the wearer may catch or snag on objects.
Seams/closures are critical components of the overall barrier protection provided by fluid-resistant or impermeable garments. It is vital to select the appropriate seam configuration to be able to protect from the penetration of blood and body fluids. Several seaming techniques are used in the construction of protective clothing, including serged or sewn, bound, taped, double taped, and ultrasonic welded. Employers should consider the barrier resistance of seams/closures when selecting the appropriate protective clothing in addition to the strength properties.
Once a facility selects a specific garment and each healthcare worker knows his or her proper garment size, switching to another supplier requires each wearer to determine the proper size needed for the specific product model selected. “ANSI/ISEA 101-2014 American National Standard for Limited-Use and Disposable Coveralls—Size and Labeling Requirements,” includes a sizing chart and a set of exercises in which a user can validate that a garment is the proper size, thereby assisting facilities in selecting the appropriate size for each wearer.
The manner in which the clothing is donned and doffed in sequence with other PPE is an important consideration when selecting gowns and coveralls. This is critical because the ease or difficulty with which PPE is put on and removed may affect its effectiveness and the potential for self-contamination, especially during doffing of contaminated PPE. Donning and doffing features included in the selection process should consider the entire PPE ensemble, not simply the gown or coverall.
In addition to the barrier resistance properties and other factors discussed above, there are other critical characteristics of protective clothing that employers and purchasers must use in their decision-making process. These include factors such as compliance with regulatory agencies, durability (abrasion resistance, tensile strength, seam strength), comfort (breathability, air permeability), flammability, electrostatic properties, cost, availability, ergonomics/human factors, and integration with other types of PPE. Of particular importance is how the selected gown or coverall will interface with other items of PPE worn by the individual healthcare worker, including gloves with the sleeve of the gown or coverall and face/eye or respiratory protective equipment with the hood or collar area of the gown or coverall. These interfaces are essential to the individual’s overall protection, because the overall ensemble of PPE provides their protection.
In selecting gowns and coveralls, further consideration should be given to the physical characteristics of the work environment and specific activities of healthcare workers. Different physical conditions where gowns or coveralls are used can compromise their material and properties of seam barriers. Certain actions, including kneeling or leaning on a chair or table contaminated with blood, can result in pressure levels that exceed the levels used in the standard test methods. The gowns or coveralls may no longer provide expected levels of protection under these conditions.
Gown and coverall manufacturers should be consulted before selections are made to:
- review both fabric and garment specifications (including type, strength, and barrier testing results of seams) of the protective clothing in consideration;
- see if the considered protective clothing is suitable for use in a medical setting;
- determine protective clothing design features, including areas of protective coverage and features that impact its ability to be effectively integrated with other forms of PPE;
- determine if a range of sizes to fit all staff is available;
- understand the ease of use (including ease of wear and removal without self-contamination); and
- review all available information on protective clothing including potential limitations, availability, and practicality.
Current Healthcare Protective Clothing Standards and Specifications
Several fluid-resistant and impermeable protective clothing options are available in the market place for healthcare workers. These include isolation gowns, surgical gowns, and coveralls. When selecting the most appropriate protective clothing, employers should consider all of the available information on recommended protective clothing, including the potential limitations. Employers should consult protective clothing manufacturers as needed in regards to availability and practicality for their facilities. A key step in this process is to understand the relevant standards and test methods. Descriptive information about each standard is provided in the body of this document.
When the transmission route is defined as “direct contact transmission,” such as in the case of Ebola and HIV, employers should consider gowns and coveralls that demonstrate resistance to synthetic blood, as well as passage of virus. Standard test methods can be used to evaluate the resistance of fabrics or seams/closures to synthetic blood penetration and viral penetration, as described in Table 1.
The United States commonly uses American Society of Testing and Materials International (ASTM) methods, while Europe commonly uses International Organization for Standardization (ISO) methods.
Table 1. Standard test methods to evaluate the resistance of fabrics to synthetic blood and virus penetration
| Barrier Property (Type of Penetration) |
ASTM Test Methods | ISO Test Methods |
|---|---|---|
| Synthetic Blood Penetration | ASTM F1670— Standard test method for resistance of materials used in protective clothing to penetration by synthetic blood. |
ISO 16603— Clothing for protection against contact with blood and body fluids—Determination of the resistance of protective clothing materials to penetration by blood and body fluids—Test method using synthetic blood. |
| Viral Penetration | ASTM F1671— Standard test method for resistance of materials used in protective clothing to penetration by bloodborne pathogens using Phi-X174 bacteriophage penetration as a test system. |
ISO 16604— Clothing for protection against contact with blood and body fluids. Determination of resistance of protective clothing materials to penetration by bloodborne pathogens— Test method using Phi-X174 bacteriophage. |
| Note: These tests are typically conducted on fabrics, but they can be conducted on the garment seams as well. It is recommended that end users inquire from the garment manufacturers about seam barrier test results, in addition to the fabrics, in order to appropriately protect healthcare workers from blood and viral penetrations. | ||
ASTM F1670 and ISO 16603 are “screening-tests” that evaluate the resistance of a material to synthetic blood penetration [ASTM 2003a; ISO 2004a]. The synthetic blood used for these tests is a mixture of cellulose, coloring, buffer solution, and stabilizing agents. Synthetic blood has a surface tension (0.042 ± 0.002 Newton per meter [N/m]) and viscosity representative of blood and some body fluids (see Table 2 for surface tension of the body fluids).
Within the context of gowns and coverall testing, the surface tension of the challenge liquid is critical. This is because liquids with higher surface tension, like water (0.070–0.072 N/m), are more likely to bead on a surface than liquids with lower surface tension, which are more likely to wet and penetrate through the garment. Consequently, some test methods that use water as a challenge agent may not be representative for evaluating the barrier effectiveness of the healthcare PPE and may overestimate the effectiveness of the PPE for blood-borne pathogens. Test methods evaluating the water resistance of garments will be discussed later in this document.
Table 2: Surface tension values for water, synthetic blood, and human blood and body fluids1
| Surface Tension (N/m) | Temperature(°C) | |||||
|---|---|---|---|---|---|---|
| Average | Min. | Max. | ||||
| Water | ||||||
| [Randall and Calman 1954] | 0.072 | — | — | 25 | ||
| Synthetic Blood | ||||||
| (used in ASTM F1670 and ISO 16603 |
0.042 ± 0.002 | — | — | 25 | ||
| Blood | ||||||
| [Attinger et al. 2013] (review) | 0.061 | — | — | 20 | ||
| [Attinger et al. 2013] (review) | — | 0.027 | 0.058 | 37 | ||
| [Hrncir et al. 1997] | 0.056 | — | — | 22 | ||
| Saliva | ||||||
| [Kazakov et al. 2009] | 0.042 | — | — | not specified | ||
| [Geigy Scientific Tables, 1984] | 0.015-0.026 | — | — | not specified | ||
| Gastric juices | ||||||
| [Spychal et al. 1990] | 0.047 | — | — | Ambient | ||
| [Aburub et al. 2008] | — | 0.035 | 0.045 | not specified | ||
| Duodenal and Jejunal fluids | ||||||
| [Fuchs and Dressman 2014] | — | 0.028 | 0.041 | not specified | ||
| Sweat | ||||||
| [Bothorel et al. 1992] | 0.0383 | — | — | 202 | ||
| [Bothorel et al. 1992] | 0.0418 | — | — | 203 | ||
| [Randall and Calman 1954] | — | 0.061 | 0.075 | 37–38 | ||
| [Geigy Scientific Tables, 1984] | 0.069-0.070 | — | — | 37–38 | ||
| 1 Vomit is usually gastric juice, although in extreme cases intestinal juices can be included. Diarrhea is just the opposite—it is predominantly intestinal juices2 Healthy
3 Atopic |
||||||
The viral penetration resistance tests, namely ASTM F1671 and ISO 16604, are similar to ASTM F1670 and ISO 16603, but they use a bacteriophage (Phi-X174) challenge suspension instead of synthetic blood [ASTM 2003a; ISO 2004b]. At the conclusion of the exposure period in the ASTM F1671 or ISO 16604 viral penetration tests, the opposing surface of the material is rinsed with an assay fluid, and this fluid is then cultured in the presence of the host bacterium, E. coli. Plaques form when a bacteriophage is present, with the number of plaques indicating the number of penetrating bacteriophages. Materials pass the viral penetration test when no liquid is observed to penetrate the specimen and the E. coli bacteriophage is not detected in the assay fluid.
The choice of virus challenge agent in the standard methods is a critical test condition. For these test methods, the bacteriophage serves as a surrogate to simulate viruses that are pathogenic to humans. Phi-X174 bacteriophage has nearly spherical morphology similar to HIV, Hepatitis B, and Hepatitis C. At 27 nm in diameter, it is similar in size and shape to Hepatitis C (30 nm in diameter), which is the smallest-known bloodborne viral pathogen.
As mentioned earlier, the size and shape of a virus are believed to affect viral penetration, and thus selecting a small virus (27 nm in diameter) would serve as a “worst-case” scenario for the barrier material. Smaller particles are expected to more easily pass through pores in the fabrics used in barrier materials. Some of the other viruses, such as Ebola virus, are larger in diameter compared to Phi-X174. Currently, there is no scientific evidence to suggest the Ebola and other larger viruses would be more likely to penetrate through protective clothing than a smaller virus.
The amount of pressure applied in the standard methods is another critical test condition. The biggest difference between the ASTM and ISO test methods is the pressure levels used when conducting test procedures. In ASTM F1670 and ASTM F1671, tests are conducted using 13.8 kilopascal (kPa) (2 pounds per square inch [psi]), and the criterion is that no penetration should occur. Whereas, in ISO 16603 and ISO 16604, the maximum pressure level before any penetration occurs is found by applying increasing pressure levels (0 kPa to 20 kPa)—14 kPa is the most equivalent pressure to that of the ASTM tests. Note that ISO 16603 and ISO 16604 are used to classify and rank materials, and they do not relate the classification of material barrier performance to any specific circumstances of use.
Penetration (often called strikethrough) can be initiated by an external force acting against clothing. The force generated by an external pressure, such as from a pressing or leaning motion, is likely one of the major routes of blood penetration, especially in the chest and sleeves of protective clothing. These pressures arise when individuals wearing protective clothing lean or press on a surface that may be wet with blood or body fluids, such as in the case of a healthcare worker leaning against a patient’s bed or an emergency medical responder kneeling on a contaminated roadway. Studies have documented a range of pressures to which protective clothing is subjected during clinical use. [Altman et al. 1991] reported that the pressures exerted on surgical gowns during pressing and leaning in surgery can range from 1 psi to 60 psi. Blood penetration has been shown to increase with increasing pressure [Granzow et al. 1998].
Although high pressures have been reported, other studies have found that many common surgical movements (including leaning, reaching, and arm resting) result in less than 2 psi pressure. For example, [Smith et al. 1995] evaluated the pressures generated during a variety of surgical procedures and found that most pressures applied to the front of surgical gowns are 2.9 psi or less for 15 seconds or less. Another study showed that leaning against the operating table caused a pressure of 0.52 psi (3.6 kPa), while reaching for an instrument showed the greatest (0.70 psi, which equals 4.8 kPa) [Smith and Nichols 1991]. The greatest pressure seen during any maneuver was 1.84 psi (12.7 kPa) while reaching. Smith and Nichols estimated representative abdominal pressures during surgical procedures to be between 0.25 and 2.0 psi.
Others have looked at the areas where blood/body fluid penetration occurs through the garment. One study found that blood penetration was most common on the chest, forearm, and abdomen, and was correlated with the areas of highest exposure and pressure [Quebbeman et al. 1992]. Others have noted that the cuff, forearm, thigh, chest, and abdomen are most vulnerable to blood strikethrough [Pissiotis et al. 1997]. Studies suggest that if a liquid is in prolonged contact with a fabric, prewetting can occur, and this can result in the fabric’s decreased resistance to penetration [Flaherty et al. 1993; Olderman 1984].
The viral penetration of surgical gowns by HIV has been compared with the soak-through point (the point at which fluid visibly soaks through the fabric) by multiple investigators [Tyler et al. 1989; Shadduck et al. 1990]. It was reported that HIV could penetrate some surgical gown materials in common use at the time of the studies, and HIV penetration was sometimes noted in the absence of visible soak-through. This is important to remember, because endusers can often have a false sense of security when they see no visible penetration in their garments.
The conditions of the ASTM F1671 test require subjecting barrier material specimens in a special test cell to the viral challenge for one hour, with the sixth minute of the exposure at 13.8 kPa (2 psi) for one minute. These conditions were selected because they are used in a related method, “ASTM F903 Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Liquids,” which assesses liquid chemical penetration through protective clothing materials. Research at Kansas State University [McCullough and Schoenberger 1992] was performed to show how these test conditions best correlated with a human factors evaluation where visible blood strikethrough occurred. This is referred to as the elbow lean test.
This technical report is structured to be as broad as possible in listing multiple test methods with minimum performance requirements in order to maximize the potential that an employer will find appropriate data from different manufacturers. However, it is important to note that different test methods, while similar, do not yield the same absolute results due to differences in test equipment, conditions, and procedures. These particular methods/standards have been selected because they are broadly used in the industry and current international protective clothing classification standards to describe the performance levels provided by garments and to differentiate the protection levels provided. Employers should be aware that garments qualifying under different standard methods may in fact provide different levels of protection. Limited information is available to compare different products using these test methods.
Standards are available to define the performance requirements for clothing or clothing materials used to protect against infectious agents. ANSI/AAMI PB70, EN 13795, EN 14126, and NFPA 1999 are examples of standards frequently used in the United States and Europe. ANSI/AAMI PB70 is used to classify the garments used in the healthcare industry, such as surgical and isolation gowns. Typically, EN 14126 is used for protective coveralls, and EN 13795 is used for surgical gowns. NFPA 1999-2013 is primarily intended for emergency medical first responders, but its scope also covers medical first receivers.
In the United States, ANSI/AAMI PB70 establishes a system of classification for protective apparel (including surgical gowns and isolation gowns) used in healthcare facilities, based on their liquid barrier performance. It also specifies labeling requirements and test methods for determining the compliance of protective apparel labeled with liquid barrier claims or liquid-borne microbial barrier claims. The ANSI/AAMI PB70 was accepted by the FDA in 2004.
The ANSI/AAMI PB70 standard includes four standard tests to evaluate the barrier effectiveness of surgical gowns, isolation gowns, and surgical drapes. Based on the results of these standardized tests, four levels of barrier performance are defined, with Level 1 being the lowest level of protection, and Level 4 being the highest level of protection. Table 3 summarizes the requirements of ANSI/AAMI PB70:2012 regarding the classification of barrier performance of surgical gowns, isolation gowns, and surgical drapes.
Table 3: ANSI/AAMI PB 70:12 classification of barrier performance of surgical gowns, other protective apparel, surgical drapes and drape accessories.
| Level1 | Test | Liquid Challenge | Result | Expected Barrier Effectiveness |
|---|---|---|---|---|
| 1 | AATCC 42 Impact Penetration2 | Water | ≤ 4.5 g | Minimal water resistance (some resistance to water spray) |
| 2 | AATCC 42 Impact Penetration | Water | ≤ 1.0 g | Low water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) |
| AATCC 127 Hydrostatic Pressure3 | Water | ≥ 20 cm | ||
| 3 | AATCC 42 Impact Penetration | Water | ≤ 1.0 g | Moderate water resistance (resistant to water spray and some resistance to water penetration under constant contact with increasing pressure) |
| AATCC 127 Hydrostatic Pressure | Water | ≥ 50 cm | ||
| 4 | ASTM F1670 Synthetic Blood Penetration Test (for surgical drapes) | Surrogate Blood | no penetration at 2 psi(13.8 kPa) | Blood and viral penetration resistance (2 psi) |
| ASTM F1671 Viral Penetration Test (for surgical and isolation gowns) | Bacteriophage Phi-X174 |
no penetration at 2 psi(13.8 kPa) | ||
| 1 In order of increasing protection2 American Association of Textile Chemists and Colorists (AATCC) 42 Water resistance: impact penetration test determines the ability of a material to resist water penetration under spray impact [AATCC 2000]
3 AATCC 127 Water resistance: hydrostatic pressure test determines the ability of a material to resist water penetration under constant contact with increasing pressure [AATCC 1998] |
||||
As indicated in Table 3, the requirements for levels 1, 2, and 3 have specific test requirements associated with them. Only Level 4 gowns are tested for viral penetration resistance, and therefore only Level 4 garments are considered impermeable to viral penetration using ASTM F1671. The gowns complying with the lower levels (Level 1, 2, and 3) cannot be considered impermeable. However, Level 1–3 gowns can provide increasing resistance to liquids. ANSI/AAMI PB70 Level 1, 2, and 3 surgical/isolation gown-testing requirements only use water as a challenge. Because the surface tension of water is much higher than that of blood, blood can penetrate through fabrics more readily than water. Therefore, no correlation can be made between AATCC water resistance tests (AATCC 42 and AATCC 127) and ASTM F1671 viral penetration tests, and consequently, protection provided by Levels 1, 2, 3 gowns and that of Level 4 gowns. A common misunderstanding among many endusers is that they are protected from blood, body fluids, and other potentially infectious materials (OPIM) when they wear any type of fluid-resistant garment or surgical or isolation gown.
The requirements for the design and construction of surgical and isolation gowns are based on the anticipated location and degree of liquid contact, given the expected conditions of use. ANSI/AAMI PB70:2012 identifies certain areas of surgical and isolation gowns as critical zones (see Figure 3). [ANSI 2012] The critical zones include those areas where direct contact with blood, body fluids, and/or OPIM is most likely to occur—even though the areas outside of critical zones can inadvertently be splashed or sprayed as well. According to the standard, for isolation gowns the whole garment is considered a critical zone due to the unpredictable types of potential contact with blood, body fluids, and OPIM. The entire isolation gown, including the seams, but excluding the cuffs, hems, and bindings, must achieve claimed barrier performance. Open-backed isolation gowns do not meet the critical area parameters, and therefore they cannot be rated. For surgical gowns, the critical zone comprises at least the front panel (area A) and lower sleeves (area B) (Figure 3). The classification of the surgical gown is based on the lower performing component of the two. According to the standard, the back of the gown (area D) can be non-protective. However, the entire front of the gown (areas A, B, and C) is required to have a barrier performance of at least Level 1. Therefore, the areas outside of critical areas in the front of gown (i.e., area C) can be Level 1 even though the whole gown is classified as Level 4 surgical gown. Therefore, using a surgical gown in isolation settings may not provide appropriate protection.
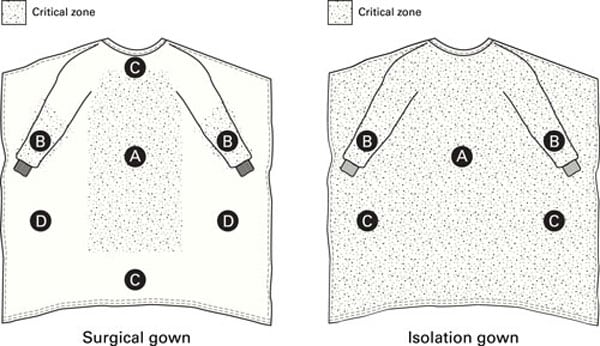
Figure 3: Critical zones defined for surgical gowns and isolation gowns in ANSI/AAMI PB70. Adapted with permission from ANSI/AAMI PB70:2012, “Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities.”
ANSI/AAMI PB70 was referenced in another standard for surgical gowns, ASTM F2407 Standard Specification for Surgical Gowns Intended for Use in Healthcare Facilities. In addition to ANSI/AAMI PB70 barrier resistance testing requirements, this standard specification also establishes physical property and comfort requirements including tensile strength, seam strength, tear resistance, lint generation, evaporative resistance, and moisture vapor transmission rate, mainly for reporting purposes only [ASTM 2407 2006].
EN 13795—“Surgical drapes, gowns and clean air suits, used as medical devices for patients, clinical staff and equipment. General requirements for manufacturers, processors and products, test methods, performance requirements and performance levels.”
EN 13795 is a European recognized standard of quality and conformance to manufacturing, testing and performance specifications for single-use and multiple-use surgical gowns, drapes, and clean-air suits. The standard was developed to assist healthcare professionals in making informed decisions when selecting the appropriate product for the anticipated tasks by specifying a consistent basis for testing and providing a common understanding of barrier properties. EN 13795 categorizes products by performance type: high performance versus standard performance gown classes. It then further subdivides them into critical and less critical product areas as in ANSI/AAMI PB70 (critical zones in surgical gowns).
EN 13795 also describes the standardized and harmonized barrier test methodologies that single-use and multiple-use surgical gowns must undergo. Table 4 shows EN 13795 specific performance requirements for surgical gowns. In addition to these tests, EN 13795 requires other characteristics be tested, including microbial and particulate matter cleanliness, linting, bursting strength (dry and wet), and tensile strength (dry and wet). However, performance requirements for these characteristics are the same for both standard-performance and high-performance surgical gowns, and critical and less-critical areas.
Note that EN 13795 is aimed to protect the patient; therefore, within the context of the European standard, the products conforming to this standard are considered medical devices. If a product is designed to be PPE rather than a medical device, the product has to comply with the corresponding directive 89/686/EEC (protective clothing) and standard “EN 14126 Performance requirements and tests methods for protective clothing against infective agents.” EN 14126 has various classes for most performance requirements; therefore, if EN 14126 is specified, then the classes for the performance requirements should also be specified.
Table 4. Overview of some of the EN 13795 performance requirements
| Characteristic | Test Method | Unit | Standard Performance | High Performance | ||
|---|---|---|---|---|---|---|
| Critical product area |
Less critical product area |
Critical product area |
Less critical product area |
|||
| Resistance to liquid penetration | EN 0811 | cm H2O | ≥20 | ≥10 | ≥100 | ≥10 |
| Resistance to microbial penetration— dry | EN ISO 22612 | CFU | N/A | ≤3001 | N/A | ≤3001 |
| Resistance to microbial penetration—wet | EN ISO 22610 | IB | ≥2.8 | N/A | 6.02 | N/A |
| 1 Test conditions: challenge concentration 108 CFU/g talc. and 30 min vibration time.2 IB = 6.0 for the purpose of this European Standard means: no penetration. IB = 6.0 is the maximum achievable value. | ||||||
Similar to “AATCC 127, Water resistance: hydrostatic pressure test,” “EN 20811 Textiles. Determination of resistance to water penetration. Hydrostatic pressure test” evaluates the behavior of the fabric structure under constantly increasing hydrostatic pressure. The results are expressed in water column (cm), and higher values indicate a better resistance. Note that ANSI/AAMI PB70 also cites this method (AATCC 127) for one of the classification requirements.
The EN ISO 22612 test method for resistance to dry microbial penetration is designed to determine the ability of dry fabric to resist penetration of particles carrying microorganisms. The method establishes the quantity of microorganisms that can penetrate through the test material while being carried on talcum powder. Test results are expressed in colony forming units (CFU) observed on the agar plate.
The EN ISO 22610 test method, used to determine the resistance to wet bacterial penetration, evaluates fabric’s resistance to microbial penetration under conditions of liquid pooling on the fabric and mechanical rubbing. Test results are expressed in IB “Barrier Index.” IB=6.0 is the maximum achievable value, and it means no penetration for the purpose of the test.
EN 14126—“Performance requirements and tests methods for protective clothing against infective agents.”
The European standard EN 14126 defines performance requirements for materials in protective clothing used to protect from infectious agents. Due to the heterogeneity of microorganisms (in terms of size, shape, infectious dose, survival abilities, etc.), the EN 14126 standard does not define performance criteria for specific types of microorganisms. The test methods specified in this standard focus on the medium containing the microorganism, such as liquid, aerosol, or solid dust particle.
The EN 14126 standard is typically used for coveralls and it specifies ISO 16603 and ISO 16604 as test methods used to evaluate the penetration resistance performance of clothing materials to contaminated liquids under hydrostatic pressure. Clothing materials are classified based on the performance levels achieved by these test methods (see Table 5). Note that the pressure level in Class 5 is closest to the pressure levels specified in ASTM F1670 and ASTM F1671 (13.8 kPa).
Note that EN 14126 specifications for ISO 16603 and ISO 16604 apply only to fabrics used in the garments. Both tests could be conducted on the seams as well. In order to be appropriately protected from blood and viral penetrations, end users should consult garment manufacturers about the seam barrier test results, in addition to the fabric test results.
Table 5: Classification according to EN 14126 of resistance to penetration by blood and body fluids using ISO 16603 and ISO 16604 test methods
| Class | Hydrostatic pressure at which material passes the test (kPa) |
|---|---|
| 6 | 20 |
| 5 | 14 |
| 4 | 7 |
| 3 | 3.5 |
| 2 | 1.75 |
| 1 | 0 |
NFPA 1999 was specifically developed to address a range of different clothing items worn by emergency medical service first responders, but also applies to medical first receivers. The standard includes design criteria, performance criteria, labeling requirements, and test methods that address both single-use (disposable) and multiple-use (reusable) emergency medical garments, which can be coveralls, multi-piece clothing sets, or partial body clothing. The standard uses ASTM F1671 to demonstrate the viral penetration resistance of materials and seams, which is supplemented with an overall liquid integrity test for full body clothing. The latter test shows whether closures and other aspects of the clothing item design will hold out liquid. There are also testing requirements applied to materials and seams for setting minimum criteria such as strength and physical hazard resistance. The standard further specifies that compliant clothing items be labeled as compliant to the standard and certified by an independent certification organization.
Several test methods and performance requirements for barrier resistant gowns and coveralls were discussed in this technical document. Test methods for determination of the barrier resistance of fabrics such as AATCC 42, AATCC 127, ASTM F1670, ASTM F1671, ISO 16603, ISO 16604, are used for both coveralls and gowns. In general, for the classification of the protective clothing, ANSI/AAMI PB70 and EN 13795 are used for gowns and EN 14126 and NFPA 1999 are used for coveralls.
There are several differences between ANSI/AAMI PB70 and EN 13795 surgical gown classifications. Because the test methods and performance requirements cannot be compared directly, it is difficult to assign equivalency between surgical gowns classified according to ANSI/AAMI PB70 (see Table 3) and EN 13795 (see Table 4). Recent PPE specifications from the World Health Organization for Filovirus disease outbreak indicate that EN 13795 high performance level gown is most similar to ANSI/AAMI PB70 level 3 gown.
Similarly, for coveralls it is difficult to compare test methods and performance specifications directly. In Europe, the EN 14126 standard typically is used to evaluate and classify coveralls used to protect from infectious agents and EN 13795 is used to evaluate and classify surgical gowns. Unlike surgical or isolation gowns (ANSI/AAMI PB70), there is no widely used classification standard in the United States. Coveralls with materials and seams tested against ASTM 1671 are specified in NFPA 1999–2013, Standard on Protective Clothing for Emergency Medical Operations. This standard establishes minimum performance requirements for single-use emergency medical garments, multiple-use emergency medical garments, and other PPE for protection from contact with blood and body-fluid-borne pathogens for personnel performing patient care during emergency medical operations [NFPA 2013]. While originally designed for pre-hospital healthcare workers, it could be used for hospital-based healthcare workers as well.
Thus, the current best approach to comparing coveralls is to use manufacturer supplied test data. Many of the same test methods used to estimate barrier protection of gowns (Tables 3 and 4) can be used for coveralls as well. Table 6 lists some of the commonly used test methods for determining the barrier effectiveness of coveralls and describes how the results should be interpreted.
Table 6: Commonly used test methods for determination of barrier effectiveness of coveralls
| Test | Challenge | Determination | Interpretation of Results |
|---|---|---|---|
| AATCC 42 Impact Penetration |
Water | Determines the ability of a material to resist water penetration under spray impact | Lower results (grams of weight gain in blotter) mean more resistant material to water penetration |
| AATCC 127 Hydrostatic Pressure | Water | Determines the ability of a material to resist water penetration under constant contact with increasing pressure | Higher hydrostatic pressure results (in water column cm or inches) mean more resistant material to water penetration |
| EN 20811 Hydrostatic Pressure | Water | Determines the ability of a material to resist water penetration under constant contact with increasing pressure | Higher hydrostatic pressure results (in water column cm or inches) mean more resistant material to water penetration |
| EN ISO 22612 Resistance to microbial penetration—dry | Contaminated (Bacillus Subtilis) talcum powder |
Determines the ability of dry fabric to resist penetration of particles carrying microorganisms | Lower colony forming units (CFU) means more resistant material to dry microbial penetration |
| EN ISO 22610 Resistance to microbial penetration—wet | Staphylococcus aureus suspension | Determines a fabric’s resistance to penetration of bacteria in a liquid while being subjected to mechanical rubbing | Number of colonies formed after incubation, expressed in Barrier Index (IB), and higher IB means more resistant material to wet microbial penetration (IB=6.0 is the maximum achievable value, it means no penetration) |
| ASTM F1670 Synthetic Blood Penetration Test | Surrogate Blood | Determines the ability of a material to resist the penetration of synthetic blood under constant contact | “Pass” means material is resistant to synthetic blood penetration at 2 psi (13.8 kPa) pressure |
| ISO 16603 Synthetic Blood Penetration Test | Material passing this test at higher pressure level is (kPa) considered more resistant to synthetic blood penetration at the specified pressure level (pressure range: 0 kPa to 20 kPa) | ||
| ASTM F1671 Viral Penetration Test | Bacteriophage (Phi-X174) challenge suspension |
Determines the ability of a material to resist the penetration of a microorganism under constant contact | “Pass” means material is resistant to viral penetration at 2 psi (13.8 kPa) pressure |
| ISO 16604 Viral Penetration Test | Material passing this test at higher pressure level (kPa) is considered more resistant to viral penetration at the specified pressure level (pressure range: 0 kPa to 20 kPa) |
AAMI [2005]. AAMI-TIR 11 Technical information report: selection of surgical gowns and drapes in healthcare facilities. Arlington, VA: Association for the Advancement of Medical Instrumentation.
AATCC [1998]. AATCC 127-1998 Water resistance: hydrostatic pressure test. Research Triangle Park, NC: American Association of Textile Chemists and Colorists.
AATCC [2000]. AATCC 42-2000 Water resistance: impact penetration test. Research Triangle Park, NC: American Association of Textile Chemists and Colorists.
Aburub A, Risely DS, Mishra D [2008]. A critical evaluation of fasted state simulating gastric fluid (FaSSGF) that contains sodium lauryl sulfate and proposal of a modified recipe. Int J Pharm 347(1–2):16–22.
Altman KW, McElhaney JH, Moylan JA, Fitzpatrick KT [1991]. Transmural surgical gown pressure measurements remits in the operating theater. Am J Infect Control 19(3):147–155.
ANSI/AAMI [2012]. ANSI/AAMI:PB70:2012 Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities. Arlington, VA: Association for the Advancement of Medical Instrumentation.
ANSI/ISEA [2014]. 101-2014 American national standard for limited-use and disposable coveralls—size and labeling requirements. Washington, DC: American National Standards Institute.
ASTM [2003a]. ASTM F1670-03 Standard test method for resistance of materials used in protective clothing to penetration by synthetic blood. West Conshohocken, PA: ASTM International.
ASTM [2003b]. ASTM F1671-03 Standard test method for resistance of materials used in protective clothing to penetration by blood-borne pathogens using Phi-X174 bacteriophage penetration as a test system. West Conshohocken, PA: ASTM International.
ASTM F2407 [2006] Standard Specification for Surgical Gowns Intended for Use in Healthcare Facilities. West Conshohocken, PA: ASTM International
Attinger D, Moore C, Donaldson A, Jafari A, Stone HA [2013]. Fluid dynamics topics in bloodstain pattern analysis: comparative review and research opportunities. Forensic Sci Int 231(1–3):375–396.
Bothorel B, Heller A, Grosshans E, Candas V [1992]. Thermal and sweating responses in normal and atopic subjects under internal and moderate external heat stress. Arch Dermatol Res 284(3):135–140.
Brown PL [1992]. Protective clothing for health care workers: liquidproofness versus microbiological resistance. In: McBriarity J, Henry N, eds. Performance of protective clothing ASTM STP 1133. Philadelphia, PA: American Society for Testing and Materials, pp. 65–82.
BSI [1992]. EN 20811: 1992 Textiles. Determination of resistance to water penetration. Hydrostatic pressure test. London: British Standards Institution.
BSI [2003]. EN 14126:2003 Protective clothing—performance requirements and tests methods for protective clothing against infective agents. London: British Standards Institution.
BSI [2011]. EN 13795:2011 Surgical drapes, gowns and clean air suits, used as medical devices for patients, clinical staff and equipment—general requirements for manufacturers, processors and products, test methods, performance requirements and performance levels. London: British Standards Institution.
Flaherty AL, Wick TM [1993]. Prolonged contact with blood alters surgical gown permeability. Am J Infect Control21(5):249–256.
Fuchs A, Dressman JB [2014]. Composition and physicochemical properties of fasted-state human duodenal and jejunal fluid: a critical evaluation of the available data. J Pharm Sci 103(11): 3398–3411.
Granzow JW, Smith JW, Nichols RL, Waterman RS, Muzik AC [1998]. Evaluation of the protective value of hospital gowns against blood strike-through and methicillin-resistant Staphylococcus aureus penetration. Am J Infect Control26(2):85–93.
Hrncir E, Rosina J [1997]. Surface tension of blood. Physiol Res 46(4): 319–321.
ISO [2004a]. ISO 16603:2004 Clothing for protection against contact with blood and body fluids—determination of the resistance of protective clothing materials to penetration by blood and body fluids—Test method using synthetic blood. Geneva, Switzerland: International Organization for Standardization.
ISO [2004b]. ISO 16604:2004 Clothing for protection against contact with blood and body fluids—determination of resistance of protective clothing materials to penetration by blood-borne pathogens—Test method using Phi-X 174 bacteriophage. Geneva, Switzerland: International Organization for Standardization.
ISO/IEC [2005]. ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories. Geneva, Switzerland: International Organization for Standardization.
Kazakov VN, Udod AA, Zinkovych II, Fainerman VB, Miller R [2009]. Dynamic surface tension of saliva: general relationships and application in medical diagnostics. Colloids Surf B Biointerfaces 74(2):457–461.
Kotilainen HR, Cyr WH, Truscott W [1992]. Ability of the 1000 mL water leak test for medical gloves to detect gloves with potential for virus penetration. In: McBriarty JP, Henry NW, eds. Performance of protective clothing. Vol. 4. ASTM STP 1133. Philadelphia, PA: American Society for Testing and Materials.
Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, Sow A, Renné T, Günther S, Lohse AW, Addo MM, Schmiedel S. [2014]. A case of severe Ebola virus infection complicated by Gram-negative septicemia. N Engl J Med: Epub ahead of print, 2014 Oct 22.
Lenter C, ed. [1984]. Geigy scientific tables. 8th ed. Vol. 1. Units for measurement, body fluids, composition of blood, hematology, somatometric data, 1984. West Caldwell, NJ: Farrand Press.
McCullough EA [1993]. Methods for determining the barrier efficacy of surgical gowns. Am J Infect Control 21(6):368–374.
McCullough EA, Schoenberger LK [1992]. A comparison of methods for measuring the liquid barrier properties of surgical gowns. In: McBriarty JP, Henry NW, eds. Performance of protective clothing. Vol 4. ASTM STP 1133. Philadelphia, PA: American Society for Testing and Materials, pp. 83–98.
NFPA [2013] National Fire Protection Association. NFPA 1999: Standard on Protective Clothing for Emergency Medical Operations. National Fire Protection Association, 2013.
Olderman GM [1984]. Liquid repellency and surgical fabric barrier properties. Eng Med 13(1):35–43.
Pissiotis CA, Komborozos V, Papoutsi C, Skrekas G [1997]. Factors that influence the effectiveness of surgical gowns in the operating theatre. Eur J Surg 163(8):597–604.
Quebbeman EJ, Telford GL, Hubbard S, Wadsworth K, Hardman B, Goodman H, Gottlieb MS [1992]. In-use evaluation of surgical gowns. Surg Gynecol Obstet 174(5):369–375.
Randall WC, Calman C [1954]. The surface tension of human sweat; its determination and its significance. J Invest Dermatol 23(2):113–118.
Siegel JD, Rhinehart E, Jackson M, Chiarello L. [2007] 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. American Journal of Infection Control; 35(10): S65-S164
Shadduck PP, Tyler DS, Lyerly HK, Sebastian MW, Farnitano C, Fitzpatrick KT [1990]. Commercially available surgical gowns do not prevent penetration by HIV-1. Surg Foram41:77–80.
Smith JW, Nichols RL [1991]. Barrier efficiency of surgical gowns: are we really protected from our patients’ pathogens? Arch Surg 126(6):756–763.
Smith JW, Tate WA, Yazdani S, Garcia RY, Muzik AC, Nichols RL [1995]. Determination of surgeon-generated pressures during various surgical procedures in the operating room. Am J Infect Control 23(4):237–246.
Spychal RT, Savalgi RS, Marrero JM, Saverymuttu SH, Kirkham JS, Northfield TC [1990]. Thermodynamic effects of bile acids in the stomach. Gastroenterology 99(2):305–310.
Thomas C, ed. [1997] Taber’s cyclopedic medical dictionary. 18th ed. Philadelphia: F.A. Davis.
Towner JS, Rollin PE, Bausch DG, Sanchez A, Crary SM, Vincent M, Lee WF, Spiropoulou CF, Ksiazek TG, Luuwiya M, Kaducu F, Downing R, Nichol ST [2004]. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 78(8):4330–4341.
Tyler DS, Lyerly HK, Nastala CL, Shadduck PP, Fitzpatrick KT, Langlois Moylan JA [1989]. Barrier protection against the human immunodeficiency virus. Curr Surg 46(4):301–304.
Balci, F. Selcen Kilinc. “Isolation gowns in health care settings: Laboratory studies, regulations and standards, and potential barriers of gown selection and use.” American journal of infection control 44.1 (2016): 104-111.
Behera BK, Arora H [2009]. Surgical gown: a critical review. J Ind Text 38(3):205–231.
Belkin NL [1994]. Gowns: selection on a procedure-driven basis. Infect Control Hosp Epidemiol 15(11):713–716.
Belkin NL [2000]. Selecting protective apparel for the degree of exposure anticipated. Infect Control Hosp Epidemiol 21(7):436.
Brown PL [1992]. Protective clothing for health care workers: liquidproofness versus microbiological resistance. In: McBriarity J, Henry N, eds. Performance of protective clothing. Vol. 4. ASTM STP 1133. Philadelphia: American Society for Testing and Materials, pp. 65–82.
Gupta BS [1988]. The effect of structural factors on the absorbent characteristics of nonwovens. Tappi J 71(8):147–152.
Henry NW III, Monteiori DG [1992]. The resistance of clothing materials to biological liquids. In: McBriarity J, Henry N, eds. Performance of protective clothing. Vol. 4. ASTM STP 1133. Philadelphia, PA: American Society for Testing and Materials, pp. 58–64.
Jaques, Peter A., et al. “Evaluation of gowns and coveralls used by medical personnel working with Ebola patients against simulated bodily fluids using an Elbow Lean Test.” Journal of occupational and environmental hygiene 13.11 (2016): 881-893.
Kilinc-Balci S [2015]. A review of isolation gowns in healthcare: fabric and gown properties. J Eng Fiber Fabr Sep; 10(3): 180–190.
Kilinc-Balci S [2014]. How well do you think you are protected? Understanding proper use and disposal of protective gowns for healthcare workers. NIOSH science blog. National Institute for Occupational Safety and Health. https://blogs.cdc.gov/niosh-science-blog/2014/05/05/gowns/. Date accessed: November 2014.
Kilinc-Balci S and D’Alessandro M [2015]. NIOSH research highlights importance of rigorous standards for gowns used to protect healthcare workers. NIOSH science blog. National Institute for Occupational Safety and Health. https://blogs.cdc.gov/niosh-science-blog/2015/07/22/isolation-gowns/ Date accessed: January 2018.
Laing RM [2008]. Protection provided by clothing and textiles against potential hazards in the operating theatre. Int J Occup Saf Ergon 14(1):107–115.
Leonas KK [2005]. Microorganism protection. In: Scott RA, ed. Textiles for protection. Boca Raton, FL: Woodhead Publishing-CRC Press, pp. 441–464.
Leonas KK, Jinkins RS [1997]. The relationship of selected fabric characteristics and the barrier effectiveness of surgical gown fabrics. Am J Infect Control 25(1):16–23.
NIOSH [2009]. Improved criteria for emergency medical protective clothing. NIOSH science blog. By Fries E, Shepherd A. National Institute for Occupational Safety and Health. https://blogs.cdc.gov/niosh-science-blog/2009/01/20/ppe-2/. Date accessed: November 2014.
Parthasarathi V, Thilagavathi G [2013]. Developing antiviral surgical gown using nonwoven fabrics for health care sector. Afr Health Sci 13(2):327–332.
Rutala WA, Weber DJ [2001]. A review of single-use and reusable gowns and drapes in health care. Infect Control Hosp Epidemiol 22(4):248–257.
Standards referenced in guidance document and imbedded links
| Standard Number | Standard Description |
|---|---|
| AATCC 127 | Water Resistance: Hydrostatic Pressure Test |
| AATCC 42 | Water Resistance: Impact Penetration Test |
| ANSI/AAMI PB70 | Liquid barrier performance and classification of protective apparel and drapes intended for use in healthcare facilities |
| ANSI/ISEA 101-2014 | American National Standard for Limited-Use and Disposable Coveralls – Size and Labeling Requirements |
| ASTM F 1671 | Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Bloodborne Pathogens Phi-X174 Bacteriophage Penetration as a Test System |
| ASTM F1670 | Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Synthetic Blood |
| ASTM F1819 | Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Synthetic Blood Using a Mechanical Pressure Technique |
| ASTM F903 | Standard Test Method for Resistance of Materials Used in Protective Clothing to Penetration by Liquids |
| CSN EN 14126 | Protective clothing. Performance requirements and tests methods for protective clothing against infective agents |
| CSN EN13795 | Surgical drapes, gowns and clean air suits, used as medical devices for patients, clinical staff and equipment. General requirements for manufacturers, processors and products, test methods, performance requirements and performance levels |
| DIN EN 20811 | Determination of Resistance To Water Penetration—Hydrostatic Pressure Test |
| EN ISO 22610 | Test method to determine the resistance to wet bacterial penetration |
| EN ISO 22612 | Test method for resistance to dry microbial penetration |
| ISO 16603 | Clothing for Protection Against Contact with Blood and Body Fluids—Determination of the Resistance of Protective Clothing Materials to Penetration by Blood and Body Fluids — Test Methods Using Synthetic Blood |
| ISO 16604 | Clothing for Protection Against Contact with Blood and Body Fluids—Determination of the Resistance of Protective Clothing Materials to Penetration by Bloodborne Pathogens — Test methods using Phi X-174 Bacteriophage |
| ISO/IEC 17025 | General requirements for the competence of testing and calibration laboratories |
| NFPA 1999 | Standard on Protective Clothing for Emergency Medical Operations |