What to know
MMR vaccine protects against three diseases: measles, mumps, and rubella.
Overview
CDC recommends children get two doses of MMR vaccine, starting with the first dose at 12 through 15 months of age, and the second dose at 4 through 6 years of age. Teens and adults should also be up to date on their MMR vaccination.
MMR vaccination is especially important for healthcare professionals, international travelers, and other specific groups.
MMR vaccine recommendations
CDC recommends children receive 2 doses of MMR vaccine.
Measles vaccine is included in MMR vaccine and MMRVA vaccine.
Rubella vaccine is included in MMR vaccine and MMRVA vaccine. Pregnant women who do not have evidence of rubella immunity should be vaccinated immediately after giving birth.
Mumps vaccine is included in MMR vaccine and MMRVA vaccine. A third dose of MMR may be recommended for people at risk of mumps during an outbreak.
About the vaccine
Three vaccines containing measles, mumps, and rubella virus are licensed for use in the United States.
- M-M-R II® is a combination measles, mumps, and rubella (MMR) vaccine manufactured by Merck & Co, Inc.
- PRIORIX® is a combination measles, mumps, and rubella (MMR) vaccine manufactured by GlaxoSmithKline Biologicals (GSK).
- ProQuad® is a combination measles, mumps, rubella, and varicella (MMRV) vaccine.
M-M-R II and PRIORIX are fully interchangeable for all indications for which MMR vaccination is recommended. All three vaccines contain live, attenuated measles, mumps, and rubella virus. MMRV also contains live, attenuated varicella-zoster virus.
The lyophilized live MMR vaccines and MMRV vaccine should be reconstituted and administered as recommended by the manufacturers123.
Vaccine effectiveness
One dose
- 1 dose of MMR vaccine is—
- 93% effective for measles (range: 39%–100%)
- 78% effective for mumps (range: 49%−92%)
- 97% effective for rubella (range: 94%–100%)
Two doses
- 2 doses of MMR are—
- 97% effective for measles (range: 67%–100%)
- 88% effective for mumps (range: 32%–95%)
People who receive MMR vaccination according to the U.S. vaccination schedule are usually considered protected for life against measles and rubella. While MMR provides effective protection against mumps for most people, immunity against mumps may decrease over time and some people may no longer be protected against mumps later in life.
- Both serologic and epidemiologic evidence indicate that vaccine-induced measles immunity appears to be long-term and probably lifelong in most persons.
- While the effectiveness of two doses of MMR against mumps is high, serologic and epidemiologic studies suggest this effectiveness decreases with time. A person with a decreased immune response after time may then become infected when exposed to mumps virus through close contact with a person with mumps. A third dose of MMR can provide added short term protection for those who are likely to have close contact with a mumps patient during an outbreak.
- Studies indicate that one dose of vaccine confers long-term, probably lifelong, protection against rubella.
The United States' long-standing vaccine safety program closely and constantly monitors the safety of vaccines. See in-depth information about the safety of each FDA-approved vaccine:
Storage and handling
Proper vaccine storage and handling practices play an important role in protecting individuals and communities from vaccine-preventable diseases. For general recommendations and guidance, see Vaccine Storage and Handling.
Three vaccines containing measles, mumps, and rubella virus are licensed for use in the United States.
- M-M-R II® is a combination measles, mumps, and rubella (MMR) vaccine.
- PRIORIX® is a combination measles, mumps, and rubella (MMR) vaccine.
- ProQuad® is a combination measles, mumps, rubella, and varicella (MMRV) vaccine.
All three vaccines must be protected from light, which might inactivate the vaccine viruses, the vaccines have different storage requirements. Administration of improperly stored vaccine might fail to provide protection against disease. The diluent can be stored in the refrigerator or at room temperature but should not be allowed to freeze. Consult package inserts for vaccine storage and handling guidance.
The Vaccine Storage and Handling Toolkit is a comprehensive resource for providers on vaccine storage and handling recommendations and best practice strategies. It includes considerations for equipment for storage units and temperature monitoring devices, strategies for maintaining the cold chain, routine storage and handling practices, inventory management and emergency procedures for protecting vaccine inventories.
Administering the vaccine
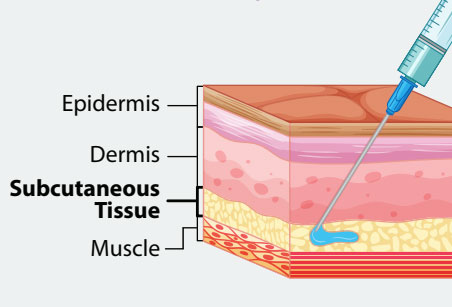
The dosage for both MMR (PRIORIX and M-M-R II) and MMRV (ProQuad) is 0.5 mL. PRIORIX is administered by the subcutaneous route only. M-M-R II and ProQuad may be administered by the subcutaneous or intramuscular route.
The minimum age for both MMR vaccines and MMRV is 12 months of age. The typical age for the second dose of either vaccine is at 4 to 6 years of age. The maximum age for administration of MMRV is 12 years. It should not be administered to anyone 13 years of age or older. Both MMR vaccines may be administered to anyone 12 months of age or older.
The minimum interval between MMR doses is 4 weeks (28 days). The minimum interval between MMRV doses is 3 months.
The preferred injection site in small children is the anterolateral aspect of the thigh. The posterior triceps aspect of the upper arm is the preferred site for older children and adolescents.
The dosage for MMR vaccine is 0.5 mL. PRIORIX is administered by the subcutaneous route only. M-M-R II may be administered subcutaneously or intramuscularly. If a second dose is indicated, the minimum interval between the first and second doses should be separated by at least 4 weeks (28 days). The preferred injection site for adults is the posterior triceps aspect of the upper arm.
- MMRV is only licensed for children 1–12 years old.
- Merck & Co. Inc. M-M-R II (Measles, mumps, and rubella virus vaccine live); 2009.
- Merck & Co. Inc. ProQuad (measles, mumps, rubella and varicella virus vaccine live lyophilized preparation for subcutaneous injection). 2011.
- GlaxoSmithKline Biologicals SA. Package insert: PRIORIX (measles, mumps, and rubella vaccine, live). 2022.