What to know
CDC’s Division of Laboratory Systems knows that incidents involving biological, chemical, physical, and radiological hazards can have a significant impact on the safety and health of those who work in laboratory settings.
How is it conducted?
Overview of the risk assessment process
In general, risk assessments can be broken down into Steps 1-2 in the figure above. The risk assessment should include considerations about the hazards (e.g., biological agent), the specific processes and procedures, existing control measures, the facility and testing environment, and the competency of the testing personnel.
Step 1: Identify the hazards and risks
In this section, learn how to answer these questions:
- What, where, and how is the work occurring?
- Who is involved in the work?
- What can go wrong?
For a specific activity or procedure, identify the hazards in each step or task that must be completed. Ask what, where, and how the work is occurring and who is doing the work. Then, determine what could go wrong in every step of the activity or procedure and the result of the undesirable incident (e.g., injury, exposure, infection, disease). One method of accomplishing this is to perform a job hazard analysis. Examples are depicted below.
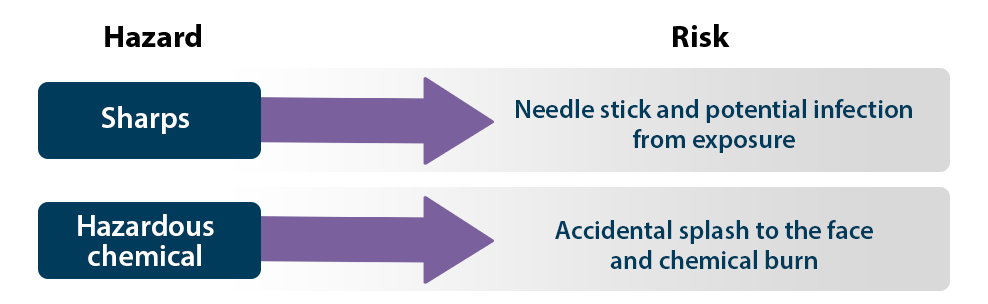
Step 2: Evaluate the risks
In this section, learn how to answer these questions:
- How likely is a risk and how severe is it?
- Is the risk acceptable or unacceptable?
a. Characterize the risks
There are various and multiple risks involved in performing laboratory testing. The risk assessment should evaluate each risk against a standard set of criteria so that the assessed risks can be compared against each other. The criteria should focus on both the likelihood of the undesirable incidents occurring and the consequences if those undesirable incidents were to occur.

Source: Sandia National Laboratory Biosafety and Biosecurity Risk Assessment Technical Guidance Document, 2014.
Likelihood and consequences of risk
The likelihood component of risk includes factors that affect whether or not the incident happens and occurs before the actual incident occurs; the consequences of risk considers factors that affect the severity of an incident after it has occurred.
It is important to define what is being evaluated because some factors can affect the likelihood and consequences. For example, the availability of appropriate personal protective equipment (PPE) can reduce the likelihood of exposure but wearing the appropriate PPE correctly can also reduce the consequences if an exposure occurs.
Likelihood of risk
Some factors to consider that can affect the likelihood of an undesirable incident (such as exposure to a biological agent in this example) include:
- Biological agent factors
- Stability in the environment (e.g., ability to produce spores, resistance to disinfectants)
- Potential routes of transmission (direct mucosal contact, inhalation, ingestion, injection)
- Endemicity of biological agent in the local environment and population (e.g., endemic or exotic) and host range
- Life stage/form of the biological agent (e.g., dimorphic fungi, antigenic shift)
- Communicability
- Stability in the environment (e.g., ability to produce spores, resistance to disinfectants)
- Laboratory/testing environment factors
- Physical infrastructure and existing controls: the type of facility, presence of engineering/safety controls, type of equipment used, function/reliability of ventilation systems
- Procedural: existence of administrative controls such as policies and training; availability of appropriate PPE; generation of aerosols and use of sharps; amplification of the biological agent by culturing, and the types and complexity of procedures being conducted
- Physical infrastructure and existing controls: the type of facility, presence of engineering/safety controls, type of equipment used, function/reliability of ventilation systems
- Human factors
- Competency of personnel, level of training
- Behavioral aspects
- Stress, risk perception, risk tolerance
- Following safe work practices
- Stress, risk perception, risk tolerance
- Competency of personnel, level of training
To evaluate the consequences after an undesirable incident occurs, assess the characteristics of the hazard(s) or biological agents, the health and immune status of the laboratory/testing personnel, and the availability of vaccines, prophylaxis, or therapies.
Consequences of risk
Some factors to consider that can affect the consequences of an undesirable incident (such as infection in this example) include:
- Biological agent factors
- Virulence factors: adhesion, invasiveness, toxigenesis, production of exoenzymes, antigenic variation, resistance to antibiotics, tissue tropism, multiple replication sites within-host, ability to elicit autoantibodies against host)
- High communicability
- Severity of infection/disease (morbidity/mortality rate)
- Infectious dose
- Virulence factors: adhesion, invasiveness, toxigenesis, production of exoenzymes, antigenic variation, resistance to antibiotics, tissue tropism, multiple replication sites within-host, ability to elicit autoantibodies against host)
- Administrative controls
- Availability of vaccines, prophylaxis, therapeutic interventions, and emergency response procedures
- Availability of vaccines, prophylaxis, therapeutic interventions, and emergency response procedures
- Host factors
- Health and immune status of staff: immunocompetent or immunocompromised, pregnancy, pre-existing medical conditions, allergies, age, large susceptible population
- Behavioral aspects
- Willingness to accept vaccines
- Adherence to safe work practices and proper use of PPE
- Willingness to accept vaccines
- Health and immune status of staff: immunocompetent or immunocompromised, pregnancy, pre-existing medical conditions, allergies, age, large susceptible population
b. Prioritize the risks and determine if risks are acceptable
It is important to acknowledge that risks can be reduced, but generally cannot be completely eliminated unless the work is discontinued entirely (e.g., elimination) or modified to incorporate less harmful activities such as using surrogates (e.g., substitution).
The risk assessment team should use the results to determine which risks are relatively higher or lower than other risks. Based on the risk assessment, the institution/testing site should determine which risks are acceptable (work can proceed with the existing controls), and which risks are unacceptable (work cannot proceed until additional mitigation controls are implemented to reduce the risk to an acceptable level).
Steps 3-4: Implement a risk mitigation plan
For risks that are determined unacceptable by the institution, a mitigation control plan should be implemented.
Step 5: Evaluate effectiveness of controls
The effectiveness of implementing additional controls (e.g., engineering controls, administrative and work practice controls, and use of PPE) should be reviewed and evaluated.
For more information on mitigation and evaluation of the performance of controls, see Biosafety in Microbiological and Biomedical Laboratories (BMBL) (6th Edition).
Resources
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition, Appendix N—Clinical Laboratories (pages 529 – 543)
- Biological Risk Assessment: General Considerations for Laboratories
- Risk Assessment: The Foundation of Every Good Biorisk Management System
- APHL Ebola Risk Assessment Template
- APHL Zika Risk Assessment Template
- APHL Risk Assessment Best Practices
- ISO 35001 Laboratory biorisk management system for laboratories and other related organizations; note that users will have to purchase the standard to view the full document
- CWA 15793 Laboratory biorisk management
- Introduction to Laboratory Risk Management (LRM)
Contact us
For more information about this Division of Laboratory Systems biorisk assessment resource, contact DLSinquiries@cdc.gov.
