What to know
Through, federal and state agencies, professional societies, and the Division of Laboratory System’s (DLS) work with other CDC programs international organizations, DLS supports the development and adoption of standards, guidelines, recommendations, and tools for improved quality and safety in clinical and public health laboratories.
Background
The following are useful resources to establish or strengthen biosafety practices in a clinical or public health laboratory.
The Centers for Disease Control and Prevention (CDC), or the Department of Health and Human Services (HHS) cannot attest to the accuracy of a non-federal site and the listing of non-federal resources and tools below does not constitute an endorsement by HHS or any of its employees of the sponsors of the information or products.
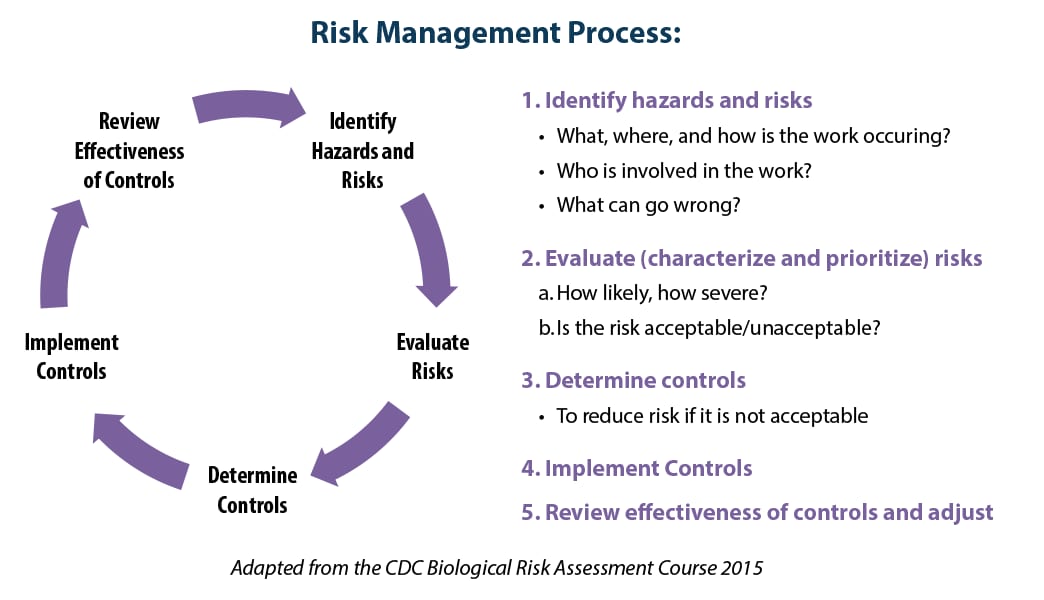
Find DLS's new biological risk management resource.
Laboratory Outreach Communication System (LOCS) Calls
Laboratory Biosafety Guidance
Questions
Contact us at DLSinquiries@cdc.gov
Disclaimer
The Centers for Disease Control and Prevention (CDC), or the Department of Health and Human Services (HHS) cannot attest to the accuracy of a non-federal site and the listing of non-federal resources and tools below does not constitute an endorsement by HHS or any of its employees of the sponsors of the information or products.
