What to know
CDC’s Division of Laboratory Systems knows that incidents involving biological, chemical, physical, and radiological hazards can have a significant impact on the safety and health of those who work in laboratory settings
What is it?
Risk management is a continuous process to identify, assess (evaluate), control, and monitor risks. The risk assessment components of the overall risk management process are:
Risk Management Process
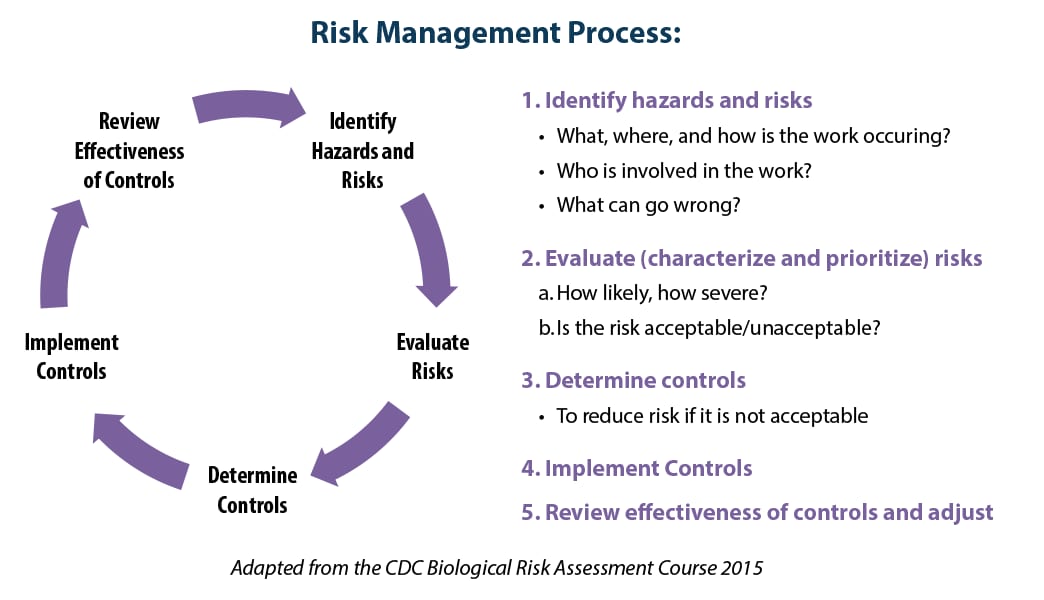
See ISO 35001 for the complete risk management process.
Process Steps
Step 1: Identify the hazards and risks.
Step 2: Evaluate the risks.
Steps 3-4: Implement a risk mitigation plan, as needed.
Step 5: Evaluate effectiveness of controls.
Why is it needed?
Many laboratory activities have been linked to undesirable events, including laboratory-acquired infections. These can result from direct contact of the infectious agent with mucous membranes of the eyes, nose or mouth via sprays, splashes, or droplets; inhalation of infectious aerosols generated during activities such as mixing and centrifugation; or from percutaneous inoculation via sharps, needle sticks, or non-intact skin (e.g., scratches and cuts).
To minimize risks and provide a safe work environment, a risk assessment should be performed to evaluate what could go wrong by determining the likelihood that an undesirable incident (e.g., injury, exposure) may occur and the consequences (e.g., infection or disease) if that undesirable incident were to occur.
When is it performed?
Formal risk assessments should be performed before work begins, and repeated when any change is introduced into the activity (e.g., changes in practices, personnel, instrumentation, or facilities). Informal risk assessments, which include short discussions among staff about current risks and mitigations, should occur much more frequently, ideally daily.
Who is involved?
A team should perform risk assessments to ensure various perspectives are considered and to reduce bias. This team could be comprised of senior leadership, clinical laboratory scientists, safety professionals, facility engineers, and others familiar with the site-specific and activity-specific laboratory and testing activities.
Contact us
For more information about this Division of Laboratory Systems biorisk assessment resource, contact us at DLSinquiries@cdc.gov.
