What to know
- An RSV antibody (nirsevimab) is available for babies and some young children to protect them from severe RSV.
- An RSV maternal vaccine (Pfizer Abrysvo) is also available for pregnant mothers at weeks 32-36 of pregnancy to pass on protection to their baby.

How to protect your children from RSV
CDC recommends RSV immunizations during specific months to maximize protection during RSV season.
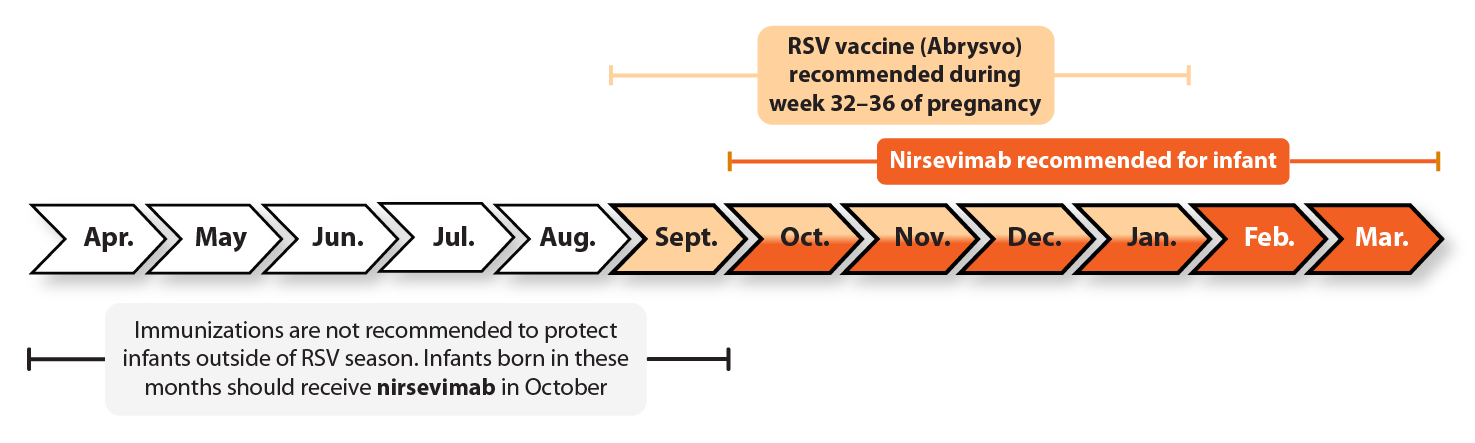
For more information, click on the tabs below.
RSV preventive antibody products
Nirsevimab is recommended for:
- All infants younger than 8 months of age born during RSV season or entering their first RSV season. Except in rare circumstances, most infants younger than 8 months of age do not need nirsevimab if they were born 14 or more days after their mother got an RSV vaccine.
- Some children ages 8 through 19 months who are at increased risk for severe RSV disease and entering their second RSV season.
Who should get nirsevimab?
Nirsevimab is recommended for infants younger than 8 months of age who were born during or are entering their first RSV season if:
- The mother did not receive an RSV vaccine during pregnancy.
- The mother's RSV vaccination status is unknown.
- The infant was born within 14 days of maternal RSV vaccination.
Most infants whose mothers got the RSV vaccine don't need to get nirsevimab too.
Some infants and young children 8 through 19 months of age who are at increased risk for severe RSV disease should receive nirsevimab shortly before the start of their second RSV season:
- Children who were born prematurely and have chronic lung disease.
- Children with severe immunocompromise.
- Children with cystic fibrosis who have severe disease.
- American Indian and Alaska Native children.
Children who should get nirsevimab but have not yet done so may get nirsevimab at any time during RSV season.
Who should not get nirsevimab?
Children 8 months old and older who are not at increased risk for severe RSV disease should not receive nirsevimab.
Except in rare circumstances, most infants younger than age 8 months do not need to get nirsevimab if their mothers got the RSV vaccine at least 14 days before delivery.
Infants and children with a history of serious allergic reactions to nirsevimab or any of its components should not get nirsevimab.
Infants and children with bleeding disorders such as hemophilia should get nirsevimab. But, as with all shots given into a muscle, parents should notify their child's healthcare provider so additional precautions can be taken.
Infants and children who have a moderate or severe acute illness usually should wait until they recover before getting nirsevimab. Your child's healthcare provider may decide to postpone giving nirsevimab until a future visit when your child feels better. Children with minor illnesses, such as a cold, can receive nirsevimab.
How well does nirsevimab work?
Nirsevimab reduces the risk of severe RSV disease by about 80%. One dose of nirsevimab protects infants for at least 5 months, the length of an average RSV season. Because nirsevimab does not activate the immune system, protection is most effective in the weeks right after nirsevimab is given and lessens over time. Nirsevimab does not provide long-term protection against RSV disease, but it does protect infants when they are most at risk of getting very sick from RSV. As children get older, they are less likely to get very sick from RSV.
How does nirsevimab prevent RSV disease?
Nirsevimab contains monoclonal antibodies, which are man-made proteins that protect against RSV. Though it does not activate the immune system the way an infection or vaccine would, a nirsevimab shot provides protection similar to that of a vaccine.
The protection that nirsevimab provides is called "passive immunity" because it does not come from the person's own immune system. Instead, the protection comes from antibodies produced outside a person's body.
On the other hand, the protection that vaccines provide is called "active immunity" because the antibodies are made by a person's own immune system. "Active immunity" requires a person's immune system to take action to defend itself.
What are the possible side effects of nirsevimab?
Side effects after nirsevimab were uncommon in clinical trials. The most common side effects after nirsevimab are pain, redness, or swelling where the injection was given, and a rash. No serious allergic reactions occurred in the clinical trials.
As with any immunization, there is a very remote chance that nirsevimab could cause a severe allergic reaction, other serious injury, or death.
If you have any questions about side effects from nirsevimab, talk with your child's health care provider.
If your child experienced side effects after receiving nirsevimab, it can be reported to the FDA or CDC. Your healthcare provider might file the report, or you can do it yourself by phone or through the MedWatch or VAERS websites.
- Report side effects that happen after getting nirsevimab to the MedWatch website or by calling 1-800-FDA-1088.
- If your child also received any vaccine on the same day as nirsevimab, the side effects also may be reported to VAERS, the Vaccine Adverse Event Reporting System, through the VAERS website or by calling 1-800-822-7967.
How do I pay for nirsevimab?
Vaccines for Children (VFC) program
Nirsevimab is covered by VFC, a federally funded program that provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay. Children younger than 19 years of age are eligible for the VFC Program if they belong to one or more of the following groups:
- Medicaid-eligible
- Uninsured
- Underinsured
- American Indian or Alaska Native
Private health insurance. Many private health insurance plans cover nirsevimab, but there may be a cost to you depending on your plan. Contact your insurer to find out.
Who should get the maternal RSV vaccine?
People who are 32 through 36 weeks pregnant during September through January should get one dose of maternal RSV vaccine to protect their babies. RSV season can vary around the country. If you live in Alaska, Florida, or outside the continental U.S., talk to your healthcare provider about when RSV season is expected where you live.
How well does the maternal RSV vaccine work?
When someone gets an RSV vaccine, their body responds by making a protein that protects against the virus that causes RSV. The process takes about 2 weeks. When a pregnant person gets an RSV vaccine, their protective proteins (called antibodies) also pass to their baby. So babies who are born at least 2 weeks after their mother gets RSV vaccine are protected at birth, when infants are at the highest risk of severe RSV disease. The vaccine can reduce a baby's risk of being hospitalized from RSV by 57% in the first six months after birth.
What are the possible side effects of the maternal RSV vaccine?
In the clinical trials, the side effects most often reported by pregnant people who received the maternal RSV vaccine were pain at the injection site, headache, muscle pain, and nausea.
Although not common, a dangerous high blood pressure condition called pre-eclampsia occurred in 1.8% of pregnant people who received the maternal RSV vaccine compared to 1.4% of pregnant people who received a placebo.
The clinical trials identified a small increase in the number of preterm births in vaccinated pregnant people. It is not clear if this is a true safety problem related to the RSV vaccine or if this occurred for reasons unrelated to vaccination.
To reduce the potential risk of preterm birth and complications from RSV disease, FDA approved the maternal RSV vaccine for use during weeks 32 through 36 of pregnancy while additional studies are conducted.
FDA is requiring the manufacturer to do additional studies that will look more closely at the potential risk of preterm births and pregnancy-related high blood pressure issues in mothers, including pre-eclampsia.
Severe allergic reactions to vaccines are rare but can happen after any vaccine and can be life-threatening. If you see signs of a severe allergic reaction after vaccination (hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, or weakness), seek immediate medical care by calling 911. As with any medicine or vaccine there is a very remote chance of the vaccine causing other serious injury or death after vaccination.
Adverse events following vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS), even if it's not clear that the vaccine caused the adverse event. You or your doctor can report an adverse event to CDC and FDA through VAERS. If you need further assistance reporting to VAERS, please email info@VAERS.org or call 1-800-822-7967.
If you have any questions about side effects from the maternal RSV vaccine, talk with your healthcare provider.
How do I pay for the maternal RSV vaccine?
Private health insurance
Most private health insurance plans cover the maternal RSV vaccine, but there may be a cost to you depending on your plan. Contact your insurer to find out.
Medicaid
Beginning October 1, 2023, most people with coverage from Medicaid and Children's Health Insurance Program (CHIP) will be guaranteed coverage of all vaccines recommended by the Advisory Committee on Immunization Practice at no cost to them.
Vaccines for Children (VFC) program
The maternal RSV vaccine will be covered by VFC, a federally funded program that provides vaccines to children who otherwise might not be vaccinated because of inability to pay. Pregnant teens enrolled in Medicaid will not be charged for the vaccine or administration. VFC-eligible teens not enrolled in Medicaid will get the vaccine at no charge but may be charged an administration fee. Children younger than 19 years of age are eligible for the VFC Program if they belong to one or more of the following groups:
- Medicaid-eligible
- Uninsured
- Underinsured
- American Indian or Alaska Native
