Key points
- Multiple studies show health care providers are the most trusted source of health information for their patients.
- Immunization against influenza (flu), COVID-19, and respiratory syncytial virus (RSV) remains the best way to safeguard against hospitalizations, long-term health impacts, and death.
- Educating patients about the fall and winter virus season will provide patients with a full set of tools to keep themselves and their families safe from respiratory diseases this season.
Purpose
Every year in the United States, flu, COVID-19, and RSV cause hundreds of thousands of hospitalizations and thousands of deaths during the fall and winter virus season. We now have more tools than ever before to help people protect themselves, their families, and communities:
- Updated immunizations are available for all three major fall and winter respiratory diseases – flu, COVID-19, and RSV (for groups eligible for RSV immunization).
- Washing hands and improving airflow in the places where people live and work are important to lowering risk from respiratory viruses.
- Widely available, effective treatments are available for those who get flu or COVID-19. Treatment can reduce severe illness, hospitalization, and death.
- Everyday actions like masking and physical distancing can provide an additional layer of protection.
- Tests are available that can quickly detect these respiratory viruses so patients don't delay treatment and other actions that can protect their family, friends, and coworkers.
Immunization recommendations
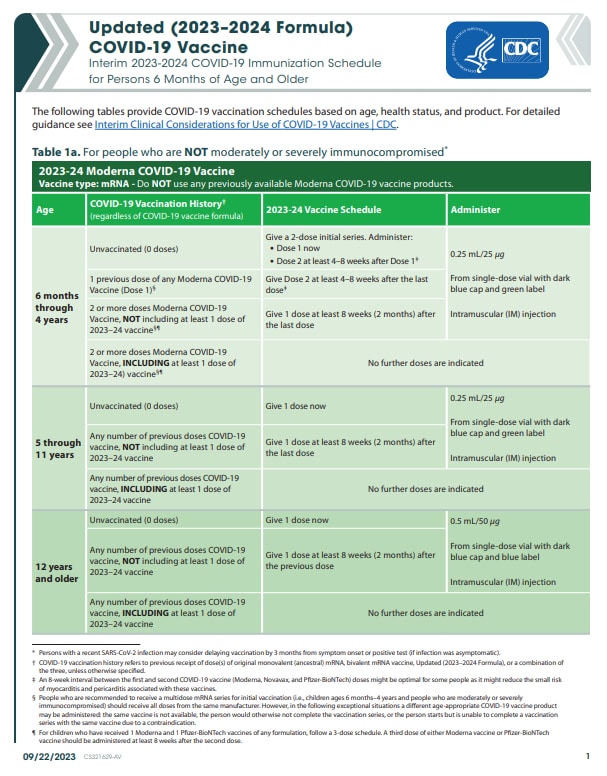
- Everyone 6 months of age and older should receive the 2023-2024 COVID-19 vaccine and the seasonal 2023-2024 influenza vaccine.
- All young infants should be protected against severe RSV disease through one of the following:
- Vaccination of pregnant people 32 through 36 weeks of gestation (Pfizer Abrysvo only) from September through January
- Immunization of infants less than 8 months born during or who are entering their first RSV season with nirsevimab, a monoclonal antibody
- Vaccination of pregnant people 32 through 36 weeks of gestation (Pfizer Abrysvo only) from September through January
- Some children aged 8 through 19 months at increased risk for severe RSV disease should receive nirsevimab when entering their second RSV season.
- Everyone 75 years of age and older should receive RSV vaccination if they haven't been previously vaccinated.
- Adults 60 through 74 years of age with certain medical conditions that increase their risk for severe RSV disease should receive RSV vaccination if they haven't been previously vaccinated.
Actions for health care providers
Before seeing the patient:
- Review immunization history and recommendations based on age, underlying medical conditions, and other risk factors. Immunization recommendations are available on the CDC website for pediatric patients and adults.
- Assess whether the patient has risk factors that place them at higher risk for severe flu and COVID-19 and should therefore receive prescription antiviral medications if they become ill.
While seeing the patient:
- Counsel the patient or caregiver that immunizations are the best way to protect themselves or their children against severe disease, hospitalization, and death from flu, COVID-19, and RSV this season. Recommend and offer immunizations to patients or caregivers or refer them to where they can get vaccines. Let patients and caregivers know they may need immunizations during their next visit.
- If a patient is eligible for COVID-19 or flu antiviral medications, explain the importance of testing and treatment early if they experience illness symptoms.
- Explain how to use the other prevention tools (testing, well-fitted mask, physical distancing, washing hands, and improving airflow or ventilation where the patient lives and works) and how these tools can help them and their families stay safe this season.
Clinical guidance
Overview
As recommended by the Advisory Committee on Immunization Practices and provided in CDC guidance:
- Healthcare providers should ideally administer nirsevimab to eligible infants and young children right before or at the start of RSV season.
- Nirsevimab may be administered outside of routine seasonal administration (i.e., October through March) based on local RSV activity and other special circumstances.
- These flexibilities should be considered to optimize patient access, including administrative procedures such as reimbursement policies for nirsevimab.
- These flexibilities should be considered to optimize patient access, including administrative procedures such as reimbursement policies for nirsevimab.
- Maternal RSV vaccination (Pfizer Abrysvo) should only be provided from September through January to pregnant people 32 through 36 weeks gestation for most of the continental United States.
- Only one product, nirsevimab or maternal RSV vaccine, is needed to protect most infants against severe RSV.
Recommendations
To protect infants and young children against severe RSV, CDC recommends administering either of the following products (only one product is necessary):
- Nirsevimab to infants aged less than 8 months and to some children aged 8 through 19 months at increased risk for severe RSV diseaseA using seasonal administration
- Maternal RSV vaccine (Pfizer Abrysvo) to pregnant persons from 32 through 36 weeks of gestation using seasonal administration
Timing of nirsevimab administration
Healthcare providers should ideally administer nirsevimab to eligible infants and young children right before or at the start of RSV season. Administration of nirsevimab should be timed to maximize protection during periods of high RSV transmission (i.e., seasonal administration from October through March in most of the continental United States). For infants born Oct. 1 to March 31, nirsevimab should be administered ideally within the first week of life. For infants born before Oct. 1 and eligible children 8 through 19 months at increased risk of severe disease, nirsevimab should ideally be administered just before or at the start of the RSV season (i.e., October or November). Only a single dose of nirsevimab is recommended for infants less than 8 months.
ACIP recommendations allow for nirsevimab to be administered outside of routine seasonal administration based on local RSV activity and other special circumstances. Nirsevimab may be indicated before October or after March for eligible infants and young children living in areas where RSV circulation is less predictable and peak activity may vary, including Alaska, and tropical climates (including but not limited to southern Florida, Guam, Hawaii, Puerto Rico, U.S.-affiliated Pacific Islands, and the U.S. Virgin Islands). State and territorial public health authorities or regional medical centers may elect to provide revised clinical and public health guidance regarding the ideal timing of nirsevimab administration based on local epidemiology and feasibility of implementation, including local supply considerations. Special circumstances may also need to be considered, such as travel to areas with increased RSV activity or concerns that the infant or child may not return for a visit when nirsevimab should ideally be administered. In the absence of local guidance or special considerations, healthcare providers should routinely administer nirsevimab to eligible infants and young children from October through March.
Flexibilities in the ACIP recommendations on the timing of nirsevimab administration should be considered to optimize patient access, including reimbursement of nirsevimab.
Timing of maternal RSV vaccine (Pfizer Abrysvo) administration
Maternal RSV vaccination (Pfizer Abrysvo) should only be provided from September through January to pregnant people 32 through 36 weeks gestation for most of the continental United States. Providers should not administer maternal RSV vaccine outside of this timeframe unless the individual lives in an area where RSV circulation is less predictable and peak activity may vary, including Alaska and tropical climates (including but not limited to southern Florida, Guam, Hawaii, Puerto Rico, U.S.-affiliated Pacific Islands, and the U.S. Virgin Islands).
If an infant requires protection against severe RSV outside of the recommended seasonal administration for maternal RSV vaccination, healthcare providers should administer nirsevimab according to the guidance provided above.
Flu, COVID-19, and RSV vaccines may be co-administered (given at the same visit). Co-administration of these vaccines might be especially important when the patient has risk factors for severe respiratory illness (including but not limited to advanced age, cardiopulmonary disease, immunocompromising conditions, and residence in a long-term care facility) and there might not be an opportunity to vaccinate the patient with all their recommended vaccines in the near future.
To optimize protection for the fall and winter virus season, providers may offer the patient all recommended respiratory virus vaccines during one visit. Patients should be aware that they may experience more side effects, like fever and fatigue, if multiple vaccines are given together; however, these side effects are generally mild or moderate and only last a day or two.
Current evidence from multiple studies supports the safety of co-administering flu and COVID-19 vaccines. There are fewer data on co-administering RSV with other vaccines; however, in clinical trials, coadministration of RSV and flu vaccines was safe. For patients at high risk of becoming seriously ill from one of these diseases, the benefits of timely protection from coadministration of more than one vaccine likely outweigh the possible risks of increased side effects.
If the provider is confident there will be additional opportunities to vaccinate the patient, and the patient prefers to receive these vaccines during different visits, there is no minimum wait period between these vaccines.
The most important thing is that patients receive all their recommended vaccines in a timely way to help protect them against these major respiratory diseases this fall and winter virus season.
A vaccine administration error is any preventable event that may cause or lead to inappropriate medication use or patient harm. Take preventive actions to avoid vaccine administration errors and establish an environment that values reporting and investigating errors as part of risk management and quality improvement.
Best practices
General Best Practice Guidelines for Immunization
Strategies to prevent errors
Vaccine Administration: Preventing Vaccine Administration Errors
RSV errors
Frequently Asked Questions About RSVpreF (Abrysvo) Vaccine for Pregnant People
COVID errors
Resources for you and your practice
Many people have questions about flu, COVID-19, and RSV immunizations. As your patients' most trusted source of health information, you play a critical role in helping them understand the importance of immunizations and that immunizations are safe and effective.
Educating staff on flu, COVID-19, and RSV immunizations will help prepare your practice for the upcoming fall and winter virus season and build trust between you and your patients.
Other vaccination materials for providers
COVID-19
U.S. COVID-19 Vaccine Product Information
RSV immunizations for infants and young children
Infant RSV Prevention At-A-Glance
Vaccine standing orders
Vaccine Standing Orders for Healthcare Providers | immunize.org
Vaccine for Children Program
CDC regularly produces educational videos and webinars to provide healthcare providers with timely and actionable information on disease activity, new clinical guidance, and immunization recommendations.

Maternal RSV Vaccination Recommendations and Implementation in Pharmacies
Other CDC videos
- Children aged 8 through 19 months at increased risk of severe RSV disease include those with chronic lung disease of prematurity who require medical support during the six months before the start of their second RSV season, severe immunocompromise, or severe cystic fibrosis; and American Indian or Alaska Native children.