Purpose
This document offers a series of strategies or options to conserve supplies of personal protective equipment (PPE) in healthcare settings when there is limited supply due to increased use and demand (e.g., as may occur during an infectious disease pandemic or epidemic) or supply chain disruption. Consideration of some contingency and crisis capacity strategies may also require authorization by the Food and Drug Administration (FDA) or enforcement discretion by the Occupational Safety and Health Administration (OSHA).
Audience
These considerations are intended for use by federal, state, and local public health officials, respiratory protection program managers, leaders in occupational health services and infection prevention and control programs, and other leaders in healthcare settings who are responsible for developing and implementing policies and procedures for preventing pathogen transmission in healthcare settings.
Hierarchy of controls
Controlling exposures to occupational hazards is a fundamental way to protect healthcare personnel (HCP) and other workers. Conventionally, a hierarchy is used to achieve feasible and effective controls. Multiple control strategies can be implemented concurrently or sequentially. This hierarchy can be represented as follows:
- Elimination
- Substitution
- Engineering controls
- Administrative controls
- Personal protective equipment (PPE)
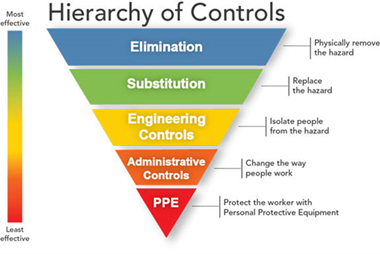
To prevent infectious disease transmission, elimination (physically removing the hazard) and substitution (replacing the hazard) are not options for healthcare settings in most circumstances. Exposures to transmissible pathogens in healthcare facilities are typically prevented through engineering and administrative controls and PPE. Prompt detection and effective triage and isolation of potentially infectious patients are essential to prevent unnecessary exposures among patients, HCP, and visitors at the facility.
Surge capacity
The greatly increased need for PPE caused by the COVID-19 pandemic caused PPE shortages, posing a tremendous challenge to the U.S. healthcare system. Healthcare facilities experienced difficulty accessing the needed PPE and had to identify alternate ways to provide patient care.
Surge capacity refers to the ability to manage a sudden increase in patient volume that would severely challenge or exceed the present capacity of a facility. While there are no commonly accepted measurements or triggers to distinguish surge capacity from daily patient care capacity, surge capacity is a useful framework to approach a decreased supply of and/or increased demand of PPE during stressors to health systems, such as public health emergencies. To help healthcare facilities plan and conserve the use of PPE in response to increased use and demand, CDC developed a Personal Protective Equipment (PPE) Burn Rate Calculator. Three general strata are used to describe surge capacity and can be used to prioritize measures to conserve PPE supplies.
- Conventional capacity: strategies consisting of engineering, administrative, and PPE controls that should or could normally be implemented in general infection prevention and control plans in healthcare settings.
- Contingency capacity: strategies that are not typically commensurate with U.S. standards of care, but may be used temporarily during periods of anticipated PPE shortages in order to prioritize and preserve PPE. Contingency capacity strategies should only be when the benefits are thought to outweigh possible harms and should not be considered a routine replacement for conventional strategies. While current supply may meet the facility's current or anticipated utilization rate, there may be uncertainty if future supply will be adequate and, therefore, contingency capacity strategies may be needed.
- Crisis capacity: strategies that are not commensurate with U.S. standards of care or worker protection, but may be used temporarily during periods of known PPE shortages. Crisis capacity strategies pose the highest risks to HCP and patient health and safety. Crisis capacity strategies should only be implemented when the benefits are thought to outweigh possible harms and should not be considered a routine replacement for conventional strategies. Facilities can consider crisis capacity strategies when the supply is not able to meet the facility's current utilization rate.
PPE supply conservations strategies
These supply conservation strategies for PPE offer a continuum of temporary options that protect HCP as much as possible when PPE supplies are stressed, running low, or exhausted. Contingency and then crisis capacity strategies augment conventional capacity strategies and are meant to be considered and implemented sequentially. Consider or implement strategies listed first before moving to strategies further down on the list. As PPE availability increases to meet needs, healthcare facilities should promptly resume standard conventional practices.
Decisions to implement contingency and crisis strategies are based on these assumptions:
- Facilities understand their current PPE inventory and supply chain and their PPE utilization rate
- Facilities are in communication with local healthcare coalitions and federal, state, and local public health partners (e.g., public health emergency preparedness and response staff) to identify additional supplies
- Facilities are routinely using conventional capacity measures
- Facilities have provided HCP with required education and training, including having them demonstrate competency with donning and doffing, with any PPE ensemble that is used to perform job responsibilities, such as provision of patient care
- Facility leaders have discussed the changes and potential risks they may pose to HCP and patients and communicated those to HCP
Healthcare facilities should promptly resume conventional capacity practices as soon as practical and by the time PPE supplies increase. These changes should be communicated to HCP. Determining the appropriate time to return to conventional strategies can be challenging. Considerations affecting this decision include:
- The number of patients for whom PPE use is recommended for their care
- Whether there is evidence of ongoing infectious disease transmission in the facility
- The incidence of the infectious disease in the community
- The number of days' supply of PPE currently remaining at the facility
- Whether or not the facility is receiving regular resupply with its full allotment
For more information
- N95 is a certification marks of the U.S. Department of Health and Human Services (HHS) registered in the United States and several international jurisdictions.
