Human Cases of Highly Pathogenic Avian Influenza A(H5N1) — California, September–December 2024
Weekly / March 13, 2025 / 74(8);127–133
Sophie Zhu, PhD1,2; Kathleen Harriman, PhD2; Caterina Liu, MD2; Vit Kraushaar, MD2; Cora Hoover, MD2; Kyoo Shim, MPH2; Sharon I. Brummitt, PhD2; Jocelyn Limas, MPH2; Kathleen Garvey, MHS2; Jennifer McNary, MPH2; Nina J. Gao, PhD2; Rahil Ryder, MS2; Brandon Stavig2; Jeffrey Schapiro, MD2; Christina Morales, PhD2; Debra A. Wadford, PhD2; Holly Howard, MPH2; James Heffelfinger, MD2; Rebecca Campagna, DVM2; Esmeralda Iniguez-Stevens, PhD2; Hamed Gharibi, PhD2; Denise Lopez, DrPH3; Laura Esbenshade3; Paula Ptomey3; Kavita K. Trivedi, MD4; Jade A. Herrera4; Joanna Locke, MD4; Nicholas Moss, MD4; Paul Rzucidlo, MPH5; Kimberly Hernandez, MPH5; Minhphuong Nguyen, MPH6; Simon Paul, MD6; Justin Mateo, MPH7; Carlos Del Carmen Luna7; Yer Chang7; Maria Rangel8; Keiryl DeLeon9; Aisha Masood9; Thea Papasozomenos, MD10; Payeng Moua10; Katie Reinhart, PhD11; Krista Kniss, MPH11; C. Todd Davis, PhD11; Marie K. Kirby, PhD11; Erica Pan, MD2; Erin L. Murray, PhD2; Los Angeles County H5 Response Team; California Department of Public Health H5 Laboratory Response Team (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Persons with occupational exposure to highly pathogenic avian influenza (HPAI) A(H5N1) virus–infected dairy cattle are at increased risk for infection.
What is added by this report?
During September 30–December 24, 2024, a total of 38 persons received a positive test result for HPAI A(H5N1) viruses in California; 37 were dairy farm workers with occupational exposure to sick cows. One, a person aged <18 years with an undetermined exposure, was the first pediatric patient detected with influenza A(H5) infection in the United States.
What are the implications for public health practice?
Public health agencies should investigate influenza-like illness or conjunctivitis in workers with occupational exposure to animals infected with HPAI A(H5N1) virus. Thorough investigations of all human HPAI A(H5N1) virus infections are necessary to identify potential exposure sources, including monitoring the virus for concerning genetic changes that indicate the potential for person-to-person transmission.
Altmetric:
Abstract
Persons who work closely with dairy cows, poultry, or other animals with suspected or confirmed infection with highly pathogenic avian influenza (HPAI) A(H5N1) viruses are at increased risk for infection. In September 2024, the California Department of Public Health was notified of the first human case of HPAI A(H5N1) in California through monitoring of workers on farms with infected cows. During September 30–December 24, 2024, a total of 38 persons received positive test results for HPAI A(H5N1) viruses in California; 37 were dairy farm workers with occupational exposure to sick cows, and one was a child aged <18 years with an undetermined exposure, the first pediatric HPAI A(H5N1) case reported in the United States. All patients had mild illness. The identification of cases associated with occupational exposure to HPAI A(H5N1) viruses on dairy farms highlights the continued risk for persons who work with infected animals. The pediatric case was identified through routine surveillance. Given recent increases in the prevalence of HPAI A(H5N1) viruses among some animal populations, public health agencies should continue to investigate cases of HPAI A(H5N1) in humans as part of control measures, pandemic preparedness, to identify concerning genetic changes, and to prevent and detect potential human-to-human transmission of the virus. To date, no human-to-human transmission of HPAI A(H5N1) virus has been identified in the United States.
Introduction
Novel influenza A virus infection, including highly pathogenic avian influenza (HPAI) A(H5N1) virus, is a reportable condition in California and nationally reportable to CDC.* In 2024, the California Department of Public Health (CDPH), California Department of Food and Agriculture (CDFA), local health departments (LHDs), and farms known to be affected by HPAI A(H5N1) (i.e., dairy or poultry farms with nonnegative [positive or inconclusive] A(H5) test results for cows, bulk milk, or poultry) coordinated to reduce infection risk and monitor HPAI A(H5N1) symptoms† among workers. All farm owners or managers of affected farms were advised to conduct daily monitoring of workers and report symptoms consistent with HPAI A(H5N1) infection in workers who were in contact with affected animals to their LHD. When farm owners did not volunteer to do the monitoring, the LHD offered to perform monitoring of symptoms directly with workers through phone calls or text messaging. Symptomatic workers were referred for specimen collection, typically, conjunctival, nasal, nasopharyngeal, or oropharyngeal swabbing, based on symptom presentation. Targeted surveillance, which includes influenza typing and subtyping for A(H5), was performed at either a local or the state public health laboratory (PHL) for all symptomatic workers or persons with epidemiologic linkage (1) to HPAI A(H5N1) reported to public health officials. PHLs use the CDC Human Influenza A Subtyping Kit which detects and differentiates hemagglutinin (H) proteins as part of routine influenza surveillance. Selected local PHLs employ the CDC Influenza A(H5) Subtyping Kit to detect A(H5)§ Asian lineage viruses for suspected HPAI A(H5N1) cases. Presumptive positive or inconclusive A(H5) specimens were sent to CDC for confirmatory testing. This report summarizes information on human HPAI A(H5N1) cases identified in California during September 30–December 24, 2024.
Investigation and Results
Initial Public Health Notification and Response
On August 30, 2024, CDFA detected, and the National Veterinary Services Laboratories subsequently confirmed, HPAI A(H5N1) virus infections in cows from three dairy farms in the Central Valley region of California. In September 2024, CDPH was notified of the first human case of HPAI A(H5N1) in California through monitoring of workers on farms with infected cows. On October 3, 2024, the first two human HPAI A(H5N1) cases in California were confirmed in workers on two separate farms where infected cows were detected in September. These patients had been identified and reported by their employers to their LHD; both had conjunctivitis, and one also had a fever. Specimens from both patients tested positive for influenza A(H5) virus at a local PHL and were confirmed as HPAI A(H5N1) at CDC. LHD staff members provided guidance on isolation and offered the antiviral oseltamivir to patients and their household members. No known epidemiologic links existed between the two patients.
As of December 24, 2024, the U.S. Department of Agriculture reported 675¶ dairy herds with infected cows, 92 commercial flocks with infected poultry,** and 35 backyard flocks with infected poultry in California. During August 30–December 24, a total of 5,126 workers were monitored at affected farms; 170 persons from 19 local health jurisdictions received testing for influenza A(H5) through targeted surveillance. One additional patient was reported through routine surveillance and subsequently received testing at a PHL. Of the 171 persons who received testing, CDPH identified 36 confirmed cases and one probable (1) case of HPAI A(H5N1) among adult dairy farm workers and one confirmed case in a child aged <18 years without dairy cow or poultry exposure; 37 persons received positive test results confirmed at CDC. This activity was reviewed by CDC and CDPH, deemed research not involving human subjects, and was conducted consistent with applicable federal law and CDC policy.††
Description of Human HPAI A(H5) Cases
Human cases with exposure to dairy cows (37). Persons with HPAI A(H5N1) infection (36 confirmed and one probable) worked at 29 unique dairy farms (Table 1). The median interval from first A(H5) virus detection in cows to the first human case on a particular farm was 7 days (range = −7 to 20 days). Worker monitoring was initiated on one unaffected farm because A(H5) virus had been detected in cows on other dairy farms owned by the same person. All patients with occupational exposure to dairy cows were aged 18–64 years (Table 2). Six patients reported underlying medical conditions. A majority (76%) worked as milkers or cared for sick cows. A majority of patients (78%) reported using personal protective equipment (PPE) at work; 25 (68%) wore gloves, 20 (54%) used eye protection (13 reported wearing goggles), 12 (32%) reported wearing boots, and six (16%) wore gowns. No patients specifically reported wearing a respirator (e.g., an N95 mask) as recommended§§; however, 12 (32%) reported wearing other face coverings or face masks.
Patients received testing a median of 2 days (range = 0–5 days) after symptom onset. All patients had mild illness. Frequently reported signs and symptoms included eye irritation or redness (97%), muscle aches (34%), and fever (29%). Respiratory symptoms, including sore throat (16%) and shortness of breath (11%) were less commonly reported. No hospitalizations or deaths occurred, and all patients recovered. All 37 patients were offered oseltamivir; two declined (5%). No cases were identified in household contacts of patients with occupational exposure.
Undetermined exposure source (one). One confirmed case was detected through routine influenza surveillance in a previously healthy child who had no known contact with infected animals or humans and had not consumed unpasteurized dairy products. This patient, who had mild respiratory symptoms and otitis media but no conjunctivitis, was not hospitalized. Oseltamivir was prescribed when positive test results were received for influenza A virus. Subtyping was positive for influenza A(H5) virus.¶¶ The patient’s three household members also had respiratory symptoms; one developed symptoms a day before the patient, while the two other members developed symptoms concurrently. Four days after the patient’s initial testing, respiratory specimens were collected from all household members. All specimens tested negative for influenza A(H5) virus. Specimens from the patient and two household members tested positive for adenovirus and rhinovirus.
Laboratory results (38). Thirty-five (95%; 37) patients received a positive conjunctival swab result, eight (28%; 29) patients received positive test results for combined nasal and oropharyngeal swabs, five (14%; 37) patients received positive nasopharyngeal swab test results, two (33%; 6) patients received positive nasal swab results, and one (25%; 4) patient received a positive oropharyngeal swab result (Table 2). The majority of patients had either a positive conjunctival or combined nasal/oropharyngeal swab (97%). One patient only received a positive nasal swab result with no other positive sites.
Genetic Sequencing
Genetic sequencing of the viruses was performed from clinical specimens of 30 patients; all were identified as HPAI A(H5N1) clade 2.3.4.4b viruses. All eight gene segments of the viruses were recovered from 16 patients, and partial gene segments were recovered from the other 14. The viruses from the 16 patients with all gene segments sequenced (Figure) were identified as HPAI A(H5N1) clade 2.3.4.4b, genotype B3.13. The pediatric patient (A/California/192/2024) only had five of eight segments sequenced, which was insufficient to classify a specific genotype; however, the neuraminidase and nucleoprotein sequences shared close genetic identity to recent California HPAI A(H5N1) B3.13 genotype viruses from humans, dairy cattle, and poultry. One virus (A/California/150/2024) contained a nucleotide substitution within the polymerase acidic gene (I38M), which is associated with reduced susceptibility to the antiviral baloxavir marboxil.*** No substitutions associated with reduced oseltamivir susceptibility or adaptations for efficient human-to-human transmission were detected.
Discussion
This report describes investigations that led to identification of 38 persons who received positive test results for HPAI A(H5N1) viruses in California; 37 were dairy farm workers with occupational exposure to sick cows, and one was a child aged <18 years with an undetermined exposure. Epidemiologic and clinical characteristics were similar to those in other U.S. human cases (2,3). In genetic sequencing of 30 of the 38 infected patients, all were identified as HPAI A(H5N1) clade 2.3.4.4b viruses. A substitution associated with reduced baloxavir susceptibility was identified in one virus sequenced from a human case in California. No additional concerning substitutions were identified.
The identification of 37 cases with occupational exposure across 29 dairy farms highlights the ongoing risk for cow-to-human transmission of HPAI A(H5N1) viruses among persons who have close contact with infected cows and their raw milk (4). The absence of cases among household contacts is consistent with the absence of viral genetic markers for efficient human-to-human transmission.
Although a majority of patients reported using PPE at work, use of recommended PPE (i.e., N95 respirators versus face mask) has been previously reported as being low among dairy farm workers with HPAI infection (5). Additional education and messaging about the risks of working with infected cows and ensuring worker access to PPE might increase PPE use, particularly if done in collaboration with farm worker organizations and producers.
This report describes the first detection of a pediatric case of influenza HPAI A(H5N1) in the United States. The source of this child’s infection remains undetermined. Unlike pediatric patients with HPAI A(H5N1) virus infections in other countries who had severe illness (6,7), this child had only mild respiratory symptoms and recovered quickly. Other sporadic cases of influenza HPAI A(H5N1) have occurred in persons with no known exposure to potentially infected animals (8). To date, human-to-human transmission of HPAI A(H5N1) viruses has not been identified in the United States.†††
Limitations
The findings in this report are subject to at least three limitations. First, information about the type of and proportion of time that PPE was worn was unavailable for all patients. Second, access to PPE was not assessed. Finally, some symptomatic persons with exposure to sick animals might not have been reported, in which case some human HPAI A(H5N1) infections might have been missed.
Implications for Public Health Practice
Public health agencies should work with dairy and poultry farms to reduce worker exposure to HPAI A(H5N1) viruses and detect and respond to human cases. Prevention, detection, and response strategies include PPE use guidance, training, and distribution; collaboration with farm managers on worker monitoring; working with LHDs to coordinate worker testing; specimen collection and laboratory testing to distinguish influenza A(H5) from seasonal influenza viruses; and distribution of oseltamivir treatment to HPAI A(H5N1) patients and oseltamivir prophylaxis to close contacts.§§§ Collaboration among public health, agriculture, animal health, occupational health, environmental health, health care providers, and other state and federal agencies is important for a coordinated One Health¶¶¶ response and to enable early detection of changes in influenza A(H5) viruses that could facilitate human-to-human transmission. Ongoing monitoring for genetic changes is necessary to assess the likelihood of antiviral resistance or human-to-human transmission of HPAI A(H5N1) viruses.
Expanded subtyping**** of influenza viruses might record additional cases of HPAI A(H5N1) virus infection with no known exposure (8). Health departments should evaluate potential exposures for all HPAI A(H5N1) cases to ascertain the possibility for human-to-human transmission. Surveillance for HPAI A(H5N1) viruses could include expanded subtyping for A(H5) testing in persons who meet epidemiologic and either clinical or public health criteria.
Acknowledgments
Persons with highly pathogenic avian influenza A(H5N1) who provided specimens and epidemiologic information; affected farm owners and managers who monitored their employees for symptoms and collaborated with public health officials; Elisabeth Burnor, Asha Choudhury, Kim Conway, Kristin Cummings, Guinevere Ellison-Giles, Sara Floor, Curtis Fritz, William Hudspeth, Chloe LeMarchand, Nancy J. Li, Christina Penton, Angela Rabe, Monica Sun, Julie Vaishampayan, Alice Yang, Alexander Yu, California Department of Public Health; Cynthia Bogert, Jennifer Book, Vanessa Cadiz, Savanna Hok, Cindy Hua, Jessica Kulow, Ha Le, Aglael Martinez Romero, Stephanie Millena, Lisa Seliskar, Tulare County Department of Health and Human Services; Kings County Department of Public Health Communicable Disease Surveillance Team; Vanessa Cardenas, Florante De Ocampo, Eric Vargas, Anthony Villa, Kern County Public Health; Josh Sanders, Merced County Public Health; Fresno County Department of Public Health Communicable Disease Investigation Team; Priyanka Anand, Megan Dorris, Sascha Ellington, Brendan Flannery, Jerome Leonard, Alexandra Mellis, Dennis Wang, 2024 Influenza A (H5N1) Response, CDC; Louise Moncla, the Nextstrain team; the Kristian Andersen Lab.
Los Angeles County H5 Response Team
Annabelle de St. Maurice, Los Angeles County Department of Public Health; Eric El-Tobgy, Los Angeles County Department of Public Health; Nicole Green, Los Angeles County Department of Public Health; Allison Joyce, Los Angeles County Department of Public Health; Cristin Mondy, Los Angeles County Department of Public Health; Taylor Mundt, Los Angeles County Department of Public Health; Heidi Ransohoff, Los Angeles County Department of Public Health; Shayra Sanchez, Los Angeles County Department of Public Health; Elizabeth Traub, Los Angeles County Department of Public Health
California Department of Public Health H5 Laboratory Response Team
Matthew Bacinskas, California Department of Public Health; John Bell, California Department of Public Health; Cynthia Bernas, California Department of Public Health; Brandon Brown, California Department of Public Health; Jahara Cayabyab, California Department of Public Health; Alice Chen, California Department of Public Health; Jesse Elder, California Department of Public Health; Shiffen Getabecha, California Department of Public Health; Carol Glaser, California Department of Public Health; Olena Gomez, California Department of Public Health; Bianca Gonzaga, California Department of Public Health; Ydelita Gonzales, California Department of Public Health; Hugo Guevara, California Department of Public Health; April Hatada, California Department of Public Health; Katya Ledin, California Department of Public Health; Deidra Lemoine, California Department of Public Health; Adrienne Macias, California Department of Public Health; Sergio Martinez-Paredes, California Department of Public Health; Blanca Molinar, California Department of Public Health; Tasha Padilla, California Department of Public Health; Chao-Yang Pan, California Department of Public Health; Kiana Pattni, California Department of Public Health; Rolando Ramirez, California Department of Public Health; Kao Saechao, California Department of Public Health; Estela Saguar, California Department of Public Health; Maria Salas, California Department of Public Health; Ioana Seritan, California Department of Public Health; Anthony Tran, California Department of Public Health; Cindy Wong, California Department of Public Health; Chelsea Wright, California Department of Public Health
Corresponding author: Sophie Zhu, uri0@cdc.gov.
1Epidemic Intelligence Service, CDC; 2California Department of Public Health; 3Tulare County Department of Health and Human Services, Visalia, California; 4Alameda County Public Health Department, San Leandro, California; 5Kern County Public Health, Bakersfield, California; 6Madera County Department of Public Health, Madera, California; 7Merced County Public Health, Merced, California; 8Fresno County Department of Public Health, Fresno, California; 9San Joaquin County Public Health Services, Stockton, California; 10Stanislaus County Health Services Agency, Modesto, California; 11Influenza Division, National Center for Immunization and Respiratory Diseases, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interests were disclosed.
* https://ndc.services.cdc.gov/conditions/novel-influenza-a-virus-infections/
† https://www.cdc.gov/bird-flu/signs-symptoms/index.html
§ CDC Human Influenza A/H5 Subtyping Kit (VER 4) Instructions for Use Package Insert. July 12, 2024.
†† 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
§§ https://www.cdc.gov/bird-flu/prevention/worker-protection-ppe.html
¶¶ https://www.cdc.gov/bird-flu/spotlights/h5n1-response-12092024.html
*** https://www.cdc.gov/bird-flu/spotlights/h5n1-response-11152024.html
††† https://www.cdc.gov/fluview/surveillance/2025-week-08.html
§§§ https://www.cdc.gov/bird-flu/prevention/hpai-interim-recommendations.html
References
- Council of State and Territorial Epidemiologists. Update to public health reporting and national notification for novel influenza A virus infection. Atlanta, GA: Council of State and Territorial Epidemiologists; 2024. https://www.cste.org/resource/resmgr/position_statements_files_2023/24-ID-09_Novel_Influenza_A.pdf
- Uyeki TM, Milton S, Abdul Hamid C, et al. Highly pathogenic avian influenza A(H5N1) virus infection in a dairy farm worker. N Engl J Med 2024;390:2028–9. https://doi.org/10.1056/NEJMc2405371 PMID:38700506
- Garg S, Reed C, Davis CT, et al. Outbreak of highly pathogenic avian influenza A(H5N1) viruses in U.S. dairy cattle and detection of two human cases—United States, 2024. MMWR Morb Mortal Wkly Rep 2024;73:501–5. https://doi.org/10.15585/mmwr.mm7321e1 PMID:38814843
- Burrough ER, Magstadt DR, Petersen B, et al. Highly pathogenic avian influenza A (H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis 2024;30:1335–43. https://doi.org/10.3201/eid3007.240508 PMID:38683888
- Marshall KE, Drehoff CC, Alden N, et al.; Colorado Field Team. Personal protective equipment use by dairy farmworkers exposed to cows infected with highly pathogenic avian influenza A(H5N1) viruses—Colorado, 2024. MMWR Morb Mortal Wkly Rep 2024;73:999–1003. https://doi.org/10.15585/mmwr.mm7344a2 PMID:39509648
- Verma A, Sharma D, Pant M, et al. First sighting of human H5N1 in Australia: a detailed account and public health implications. New Microbes New Infect 2024;60-61:101447. https://doi.org/10.1016/j.nmni.2024.101447 PMID:39045288
- Jassem AN, Roberts A, Tyson J, et al. Critical illness in an adolescent with influenza A(H5N1) virus infection. N Engl J Med 2025;392:927–9. https://doi.org/10.1056/NEJMc2415890 PMID:39740022
- Garg S, Reinhart K, Couture A, et al. Highly pathogenic avian influenza A (H5N1) virus infections in humans. N Engl J Med 2025;392:843–54. https://doi.org/10.1056/NEJMoa2414610 PMID:39740051
* Farms were quarantined until reporting no cows with signs of infection and three consecutive weekly negative tests of bulk milk; no farms with human cases were released from quarantine through December 24, 2024. Quarantine of sick cows is necessary to reduce farm-to-farm and cow-to-human transmission of highly pathogenic avian influenza A(H5N1) viruses.
† Worker monitoring was initiated on farm U because A(H5) virus had been detected in cows on other dairy farms with the same owner. The virus was detected on the farm after the first human case occurred in a farm worker.
* Table includes 37 persons with occupational exposure to infected dairy cows and one with an unknown exposure source to influenza A(H5).
† https://cdn.ymaws.com/www.cste.org/resource/resmgr/position_statements_files_2023/24-ID-09_Novel_Influenza_A.pdf
§ Race and ethnicity not described for one person to protect privacy.
¶ Some cases were confirmed with more than one specimen.
** Measured or subjective fever.
†† Farmhands and persons in the “Other” categories were in roles with close contact with sick cows.
§§ Eye protection (including goggles), gloves, gown, or boots.
 FIGURE. Phylogenetic tree* of 16 whole genome highly pathogenic avian influenza A(H5N1) viruses, by identification and collection date, from human cases — California, September–December 2024
FIGURE. Phylogenetic tree* of 16 whole genome highly pathogenic avian influenza A(H5N1) viruses, by identification and collection date, from human cases — California, September–December 2024
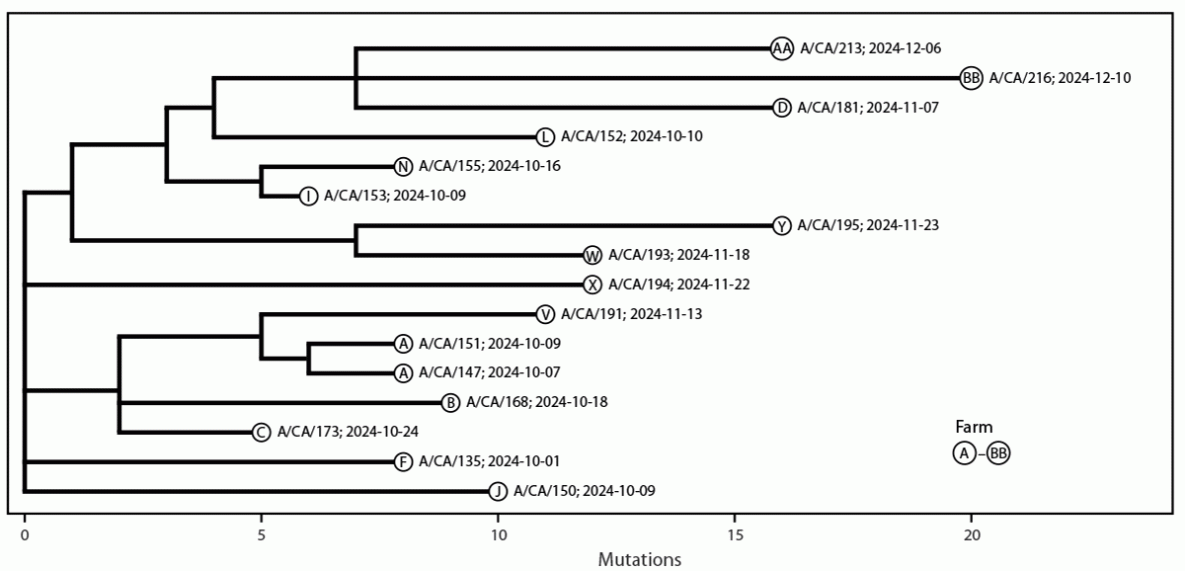
* Tree was created with Ultrafast Sample placement on Existing tRee (UShER). https://genome.ucsc.edu/cgi-bin/hgPhyloPlace and Auspice https://auspice.us/
Suggested citation for this article: Zhu S, Harriman K, Liu C, et al. Human Cases of Highly Pathogenic Avian Influenza A(H5N1) — California, September–December 2024. MMWR Morb Mortal Wkly Rep 2025;74:127–133. DOI: http://dx.doi.org/10.15585/mmwr.mm7408a1.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.