Notes from the Field: Increase in Diagnoses of Human Parvovirus B19–Associated Aplastic Crises in Children and Adolescents with Sickle Cell Disease — Atlanta, Georgia, December 14, 2023–September 30, 2024
Weekly / November 28, 2024 / 73(47);1090–1091
Marianne E.M. Yee, MD1,2; Grace G. Kalmus, MPH1; Ashwin P. Patel, MD, PhD1,2; Jason N. Payne, MD1,2,3; Amy Tang, MD1,2; Beatrice E. Gee, MD1,2,3 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Human parvovirus B19 (B19) can cause transient aplastic crises in persons with chronic anemia, including sickle cell disease (SCD).
What is added by this report?
In a large SCD center in the southeastern United States, the incidence rate of B19-associated aplastic crisis was 3.6 times higher in the first 9 months of 2024 compared with the overall rate during 2010–2023.
What are the implications for public health practice?
Health care providers should be aware of increased B19 activity in 2024 and consider B19 infection in persons with SCD presenting with anemia and reticulocytopenia because this population might require urgent blood transfusion to reduce the risk for severe complications.
Altmetric:
Human parvovirus B19 (B19) is a common viral infection transmitted by respiratory droplets. Although B19 infection is typically mild in healthy persons, persons with sickle cell disease (SCD) can experience severe anemia from impairment of red blood cell production (1–3).
In December 2023, a child aged 10 years with SCD (child A) died unexpectedly at home, with no preceding fever or symptoms. Limited laboratory testing showed hematocrit <15% (normal = 37%–45%); testing for B19 was not performed. Massive splenomegaly was noted on autopsy, consistent with splenic sequestration crisis associated with SCD. Six days later, child A’s sibling (child B), aged 14 years, who also has SCD, was confirmed to have acute B19 infection. Child B’s laboratory evaluation showed hemoglobin <6 g/dL (normal = 12.0–16.0 g/dL) and reticulocyte count 7.0 × 109/L (normal = 40–102 × 109/L), consistent with aplastic crisis. Child B received a red blood cell transfusion and recovered without complications. Data from the Sickle Cell Clinical Database of Children’s Healthcare of Atlanta (CHOA) were reviewed to describe recent trends in B19 infection among children with SCD. This study was approved by the Institutional Review Board of CHOA.
Investigations and Outcomes
At CHOA, patients with SCD are presumed to have aplastic crisis and are tested for B19 if laboratory evaluation shows a decrease in hemoglobin ≥1 g/dL from baseline with reticulocytopenia. Examination of data from CHOA’s Sickle Cell Clinical Database identified 55 cases of B19 infection with aplastic crisis in children with SCD during December 2023–September 30, 2024 (two in 2023 [child B and another, unrelated child] and 53 in 2024). During January–September 2024, the incidence of B19 infection associated with aplastic crisis among all pediatric patients with SCD who had a clinical encounter at CHOA during that calendar year was 35.6 per 1,000 patient-years, 3.6 times higher than that during 2010–2023 (7.78 per 1,000 patient-years) (Figure). Increased numbers of B19-associated aplastic crisis cases and incidence occurred in 2014 (34 cases; 19.2 per 1,000 patient-years) and 2019 (30 cases; 14.7 per 1,000 patient-years). Based on clinical suspicion, during 2010–2023, a median of 2.9% of all patients with SCD with health care encounters at CHOA were tested for B19 per year (range = 1.4%–5.7%); in 2024, 6.8% of patients were tested (Supplementary Figure, https://stacks.cdc.gov/view/cdc/170361).
The median patient age at infection since December 2023 was 10.1 years, compared with 7.7 years during 2010–January 2023 (p = 0.004).* Common signs and symptoms included pain (78%), fever (62%), fatigue (31%), and respiratory symptoms (26%). The median decline in hemoglobin from baseline to nadir was 3.6 g/dL (IQR = 3.0–4.8). Complications included acute chest syndrome† (27%), splenic sequestration§ (11%), stroke (3.6%), and nephrotic syndrome (1.8%). Forty-three (78%) patients received red blood cell transfusions. Other than child A, in whom a diagnosis of B19 was not confirmed, no other patients died with B19 infection.
Preliminary Conclusions and Actions
During 2024, an increased incidence of B19-associated aplastic crisis was observed among patients with SCD at one pediatric health care system in Atlanta, compared with incidence during 2010–2023. Among SCD patients with B19 infection, the most common initial signs of infection include anemia and reticulocytopenia. Health care providers should be aware of the risk for complications of B19 infection in children with SCD and have a low threshold for testing when there is clinical suspicion of aplastic crisis. Children and adolescents with SCD and B19 infection should be monitored for complications; early red blood cell transfusion might prevent serious adverse outcomes.
Corresponding author: Marianne E.M. Yee, memcphe@emory.edu.
1Aflac Cancer and Blood Disorders Center, Children’s Healthcare of Atlanta, Atlanta, Georgia; 2Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia; 3Department of Pediatrics, Morehouse School of Medicine, Atlanta, Georgia.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Beatrice E. Gee, Grace G. Kalmus, Jason N. Payne, Amy Tang, and Marianne E.M. Yee report support to the Sickle Cell Disease Health Services and Outcomes Program from the Abraham J. and Phyllis Katz Foundation. Marianne E.M. Yee also reports grant support from the National Institutes of Health (NIH), receipt of honoraria for a presentation at the Advanced Immunohematology & Molecular Symposium in Denver, Colorado, June 5, 2023, and from the American Board of Pediatrics, Pediatric Hematology/Oncology sub-board. Amy Tang reports grants from NIH, Pfizer, and Novo Nordisk; and service as chair of the hemoglobinopathies interest group of the American Society of Pediatric Hematology/Oncology. Jason N. Payne reports support from the American Society of Hematology for participation in the Clinical Research Training Institute. Beatrice E. Gee reports grants from NIH. No other potential conflicts of interest were disclosed.
* Wilcoxon rank-sum test.
† A vaso-occlusive complication in the pulmonary vasculature.
§ A sudden enlargement of the spleen caused by trapping of sickled red blood cells, resulting in reduction in blood volume and hemoglobin.
References
- Serjeant GR, Mason K, Topley JM, et al. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet 1981;318:595–7. https://doi.org/10.1016/S0140-6736(81)92739-2 PMID:6116082
- Tsitsikas DA, Gallinella G, Patel S, Seligman H, Greaves P, Amos RJ. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev 2014;28:23–30. https://doi.org/10.1016/j.blre.2013.12.002 PMID:24468004
- Rogers HJ, Feasel P. Acute parvovirus B19 infection detected in bone marrow biopsy. Blood 2015;126:1630. https://doi.org/10.1182/blood-2015-07-656157 PMID:26668860
 FIGURE. Human parvovirus B19–associated aplastic crisis cases (A) and incidence (B) identified among children and adolescents with sickle cell disease — Children’s Healthcare of Atlanta, Atlanta, Georgia, January 1, 2010–September 30, 2024
FIGURE. Human parvovirus B19–associated aplastic crisis cases (A) and incidence (B) identified among children and adolescents with sickle cell disease — Children’s Healthcare of Atlanta, Atlanta, Georgia, January 1, 2010–September 30, 2024
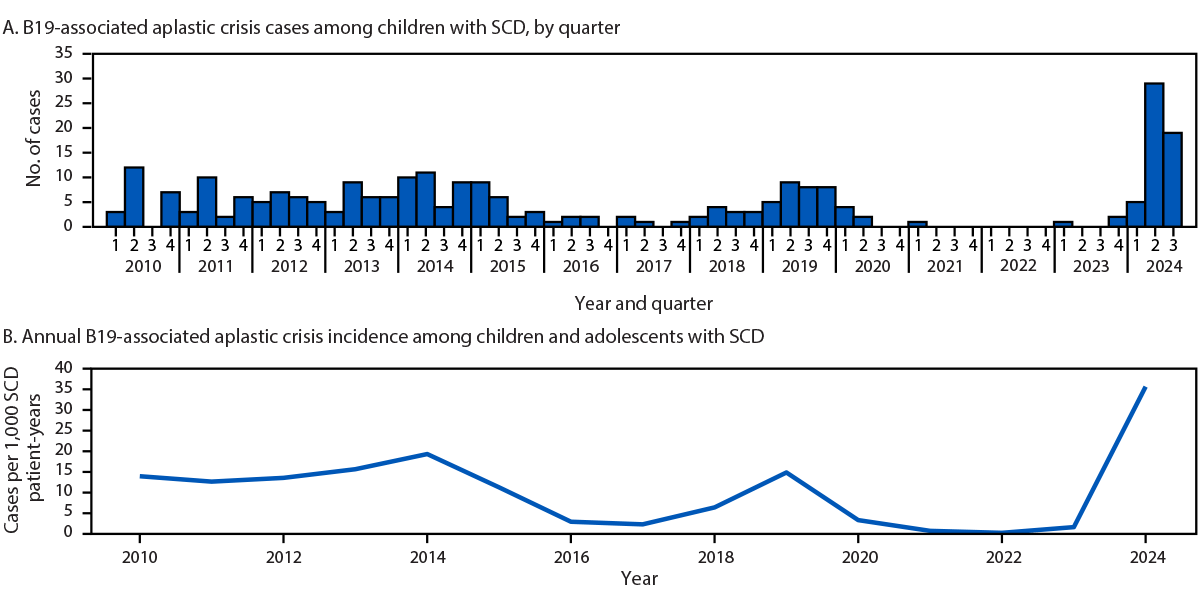
Abbreviations: B19 = human parvovirus B19; SCD = sickle cell disease.
Suggested citation for this article: Yee ME, Kalmus GG, Patel AP, Payne JN, Tang A, Gee BE. Notes from the Field: Increase in Diagnoses of Human Parvovirus B19–Associated Aplastic Crises in Children and Adolescents with Sickle Cell Disease — Atlanta, Georgia, December 14, 2023–September 30, 2024. MMWR Morb Mortal Wkly Rep 2024;73:1090–1091. DOI: http://dx.doi.org/10.15585/mmwr.mm7347a5.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.