Interim Effectiveness Estimates of 2024 Southern Hemisphere Influenza Vaccines in Preventing Influenza-Associated Hospitalization — REVELAC-i Network, Five South American Countries, March–July 2024
Weekly / October 3, 2024 / 73(39);861–868
Erica E. Zeno, PhD1,2,*; Francisco Nogareda, MPH3,*; Annette Regan, PhD3,4; Paula Couto, MD3; Marc Rondy, PhD3; Jorge Jara, MD3; Carla Voto, MD5; Maria Paz Rojas Mena, MD5; Nathalia Katz, MD6; Maria del Valle Juarez, MPH6; Estefanía Benedetti, MPH7; Francisco José de Paula Júnior, MD8; Walquiria Aparecida Ferreira da Almeida, PhD8; Carlos Edson Hott, MBA8; Paula Rodríguez Ferrari, MSN9; Natalia Vergara Mallegas, MPH9; Marcela Avendaño Vigueras9; Chavely Domínguez, MD10; Marta von Horoch, MD11; Cynthia Vazquez, Phd12; Eduardo Silvera13; Hector Chiparelli, MD14; Natalia Goni, PhD14; Laura Castro, DrPH1; Perrine Marcenac, PhD1; Rebecca J. Kondor, PhD1; Juliana Leite, PhD3; Martha Velandia, MD3; Eduardo Azziz-Baumgartner1; Ashley L. Fowlkes, ScD1; Daniel Salas, MD3; REVELAC-i Network (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Influenza vaccine effectiveness (VE) varies by season.
What is added by this report?
In five South American countries (Argentina, Brazil, Chile, Paraguay, and Uruguay) the 2024 Southern Hemisphere seasonal influenza vaccine reduced the risk for influenza-associated hospitalization among high-risk groups by 35%. VE might be similar in the Northern Hemisphere if similar A(H3N2) viruses predominate during the 2024–25 influenza season.
What are the implications for public health practice?
CDC recommends that all eligible persons aged ≥6 months receive seasonal influenza vaccine. Early antiviral treatment can complement vaccination to protect against severe influenza-related morbidity.
Altmetric:
Abstract
To reduce influenza-associated morbidity and mortality, countries in South America recommend annual influenza vaccination for persons at high risk for severe influenza illness, including young children, persons with preexisting health conditions, and older adults. Interim estimates of influenza vaccine effectiveness (VE) from Southern Hemisphere countries can provide early information about the protective effects of vaccination and help guide Northern Hemisphere countries in advance of their season. Using data from a multicountry network, investigators estimated interim VE against influenza-associated severe acute respiratory illness (SARI) hospitalization using a test-negative case-control design. During March 13–July 19, 2024, Argentina, Brazil, Chile, Paraguay, and Uruguay identified 11,751 influenza-associated SARI cases; on average, 21.3% of patients were vaccinated against influenza, and the adjusted VE against hospitalization was 34.5%. The adjusted VE against the predominating subtype A(H3N2) was 36.5% and against A(H1N1)pdm09 was 37.1%. These interim VE estimates suggest that although the proportion of hospitalized patients who were vaccinated was modest, vaccination with the Southern Hemisphere influenza vaccine significantly lowered the risk for hospitalization. Northern Hemisphere countries should, therefore, anticipate the need for robust influenza vaccination campaigns and early antiviral treatment to achieve optimal protection against influenza-associated complications.
Introduction
Influenza epidemics typically occur during the cool weather months of April–September in the Southern Hemisphere and October–May in the Northern Hemisphere. Every year, it is estimated that influenza results in 716,000–829,000 hospitalizations and 41,007–71,710 deaths throughout the Americas (1,2). To prevent influenza-associated morbidity and mortality, most countries in the Americas have implemented influenza vaccination programs (3). The Pan American Health Organization (PAHO) Network for the Evaluation of Vaccine Effectiveness in Latin America and the Caribbean – influenza (Red para la Evaluación de Vacunas en Latino América y el Caribe – influenza [REVELAC-i])† provides timely information about the vaccination status of hospitalized influenza patients and vaccine effectiveness (VE), which guides public health messaging and influenza vaccine composition decisions for each Southern Hemisphere season. Southern Hemisphere VE estimates also herald what Northern Hemisphere jurisdictions might anticipate about VE if the same influenza viruses circulate during their upcoming influenza season.
Methods
Data Sources
Patients with severe acute respiratory illness (SARI), defined as an acute respiratory illness with either a history of fever or measured body temperature ≥100.4°F (≥38°C), cough, and onset ≤10 days before hospitalization, were identified through the SARInet Plus network.§,¶ Respiratory specimens were tested for influenza virus by reverse transcription–polymerase chain reaction (RT-PCR) and typed and subtyped in national reference laboratories.
The study population comprised SARI patients in three mutually exclusive PAHO target groups for vaccination: young children, persons with comorbidities, and older adults; definitions of young children and older adults varied among the countries.** March–July 2024 data were pooled from 2,535 hospitals, including 30 in Argentina, 2,477 in Brazil, 13 in Chile, five in Paraguay, and 10 in Uruguay. VE evaluation began 2 weeks after commencement of each country’s influenza vaccination campaign.†† All countries used World Health Organization (WHO)–recommended egg-based Southern Hemisphere formulations. Argentina, Brazil, Chile, and Uruguay used trivalent vaccines containing antigens from A/Victoria/4897/2022 (H1N1)pdm09–like virus, A/Thailand/8/2022 (H3N2)–like virus, and B/Austria/1359417/2021 (B/Victoria lineage)–like virus. Paraguay used quadrivalent vaccines that also contained the B/Yamagata lineage–like virus.§§
Study Design
VE against influenza-associated hospitalization was estimated using a test-negative case-control study design. Case-patients were SARI patients who received a positive influenza RT-PCR test result. Control patients were SARI patients who received negative RT-PCR test results for both influenza virus and SARS-CoV-2 (4). Vaccination status was ascertained using unique patient identifiers to link to national electronic immunization records. SARI patients who received the 2024 influenza vaccine ≥14 days before symptom onset were considered vaccinated. Those not vaccinated before symptom onset were considered unvaccinated, and those vaccinated 0–13 days before symptom onset were excluded from the evaluation.
Data Analysis
VE was calculated by comparing the odds of influenza vaccination between influenza test-positive SARI case-patients and influenza test-negative control patients using multivariable logistic regression, overall and by target group.¶¶ To reduce potential confounding, models were adjusted for country, sex, age in years (cubic spline), week of symptom onset (cubic spline), and presence of at least one comorbidity. Analyses were stratified by influenza type and subtype when at least five patients contributed to each stratum or when the width of the 95% CI was <140 percentage points from lower to upper bounds. Because Brazil accounted for the majority of SARI cases, a sensitivity analysis excluding Brazil was conducted. This activity was reviewed by CDC, deemed not research, and conducted consistent with applicable federal law and CDC policy.***
Results
Characteristics of the Study Population
During March 13–July 19, 2024, among a total of 111,856 SARI patients identified, 100,260 were excluded because of missing influenza RT-PCR results (70,055); ineligibility or not being in a vaccine target group (14,245); symptom onset before vaccine availability, outside the influenza season, or after hospital admission (7,581); unknown vaccination status or vaccination date (5,157); specimen collection >10 days after symptom onset (1,220); vaccination <14 days before symptom onset (911); receipt of a positive SARS-CoV-2 test result (503); not meeting the SARI case definition (251); or missing demographic information (201). A total of 11,751 patients met inclusion criteria, including 630 (5.4%) from Argentina, 9,095 (77.4%) from Brazil, 1,584 (13.5%) from Chile, 162 (1.4%) from Paraguay, and 280 (2.4%) from Uruguay (Table 1). Overall, 6,851 (58.3%) patients were young children, 1,702 (14.5%) were older children and adults with comorbidities, and 3,198 (27.2%) were older adults. The majority of SARI patients in Brazil were in the young children target group.
Characteristics of Influenza Case-Patients
Approximately one third (32.7%; 3,848) of SARI patients received a positive influenza test result; most (98.6%) viruses identified were influenza A viruses. Only 26 (0.7%) patients were infected with influenza B viruses, all of which were B/Victoria lineage; influenza virus type was missing for 28 (0.7%) case-patients. Among 2,382 (61.9%) influenza A viruses that were subtyped, 1,628 (68.3%) were A(H3N2) and 754 (31.7%) A(H1N1)pdm09 (Figure). The majority of influenza case-patients were older adults (59.2%), followed by persons with comorbidities (50.4%); the lowest percentage of cases (16.0%) occurred among young children (p<0.001).
Vaccination Status of Case- and Control Patients
Overall, 21.3% of SARI patients were vaccinated; vaccination coverage varied by target group: 29.3% of older adults, 19.4% of young children, and 14.5% of persons with comorbidities were vaccinated (p<0.001) (Table 1). Among 3,848 influenza case-patients, 704 (18.3%) had received a 2024 seasonal influenza vaccine compared with 1,804 of 7,903 (22.8%) control patients (p<0.001).
Vaccine Effectiveness
The adjusted VE against any influenza-associated hospitalization was 34.5% overall, including 58.7% among persons with comorbidities, 39.0% among young children, and 31.2% among older adults (Table 2). Among influenza A subtypes, VE was 36.5% against the predominating A(H3N2) and 37.1% against A(H1N1)pdm09. As of July 19, too few influenza B detections were available to estimate VE. Adjusted VE against SARI from any influenza virus was 42.2% in Argentina, 30.3% in Brazil, 56.9% in Chile, and 61.0% in Uruguay; VE was not calculated for Paraguay because data were insufficient. In the sensitivity analysis excluding Brazil, the adjusted VE for all other countries was 56.5%.
Genetic Characterization of Viruses Reported by REVELAC-i Countries
As of August 12, most A(H1N1)pdm09 viruses reported by REVELAC-i countries to the Global Initiative on Sharing All Influenza Data††† were clade 5a.2a.1 (64.2%) or 5a.2a (35.8%). Most reported A(H3N2) viruses were clade 2a.3a.1 subclade J.2 (92.3%), subclade J.1 (7.5%), or subclade J (0.1%).§§§
Discussion
This evaluation suggests that while only one in five SARI patients had received the 2024 influenza vaccine, those who were vaccinated were at significantly lower risk for hospitalization from any influenza virus infection, including the predominant influenza A(H3N2) and influenza A(H1N1)pdm09 subtypes. Although South American countries prioritized young children, persons with comorbidities, and older adults for vaccination to prevent influenza illness complications, the documented influenza vaccination coverage levels (21.3%) were below pre–COVID-19 norms. This finding is consistent with postpandemic declines in vaccination coverage across the Americas associated with vaccine misinformation, hesitancy, and disruptions in routine immunization services, prevalent during the COVID-19 pandemic (3). Vaccination remains one of the most effective measures to prevent influenza-associated complications, including death.¶¶¶ Annual influenza vaccination should be encouraged for young children, persons with comorbidities, and older adults (5). Influenza vaccine postintroduction evaluations and knowledge, attitudes, and practices surveys might identify additional reasons for low coverage and strategies for improved coverage for the next Southern Hemisphere season.
Despite the low influenza vaccination coverage, those vaccinated were protected against hospitalization. The 34.5% REVELAC-i VE against all influenza-associated hospitalization was within historical ranges of 34%–53% against A(H3N2) and 18%–56% against A(H1N1)pdm09 (6). Vaccination likely prevented 36.5% of influenza A(H3N2)–associated and 37.1% of influenza A(H1N1)pdm09–associated hospitalizations. VE was lowest in Brazil, likely because a higher proportion of cases in Brazil occurred among young children, a population with a VE estimate in the lower range among the three target groups. If these clades predominate during the Northern Hemisphere influenza season and the updated A/Thailand/8/2022 (H3N2)–like virus antigen provides similar protection against clade 2a.3a.1, health authorities might anticipate similar levels of protection from the 2024–25 vaccine (7). To enhance this year’s modest influenza vaccine protection against hospitalization, providers should treat patients with suspected or confirmed influenza as soon as possible with antivirals.
Limitations
The findings in this report are subject to at least five limitations. First, small interim-estimate sample sizes precluded the estimation of VE against influenza B. Second, although the analyses were robust, 63% of patients were excluded because they did not receive RT-PCR results in time for the interim analysis. Third, Brazil, which is approximately three times as populous as the other countries combined,**** accounted for approximately 80% of the study population and included a higher percentage of SARI patients in the young children target group compared with that in other countries. Overall analyses were adjusted for country and target group but might still be more representative of Brazil’s VE estimate. Fourth, this analysis does not distinguish between young children who received 1 or 2 vaccine doses; VE might be higher among young children who received 2 influenza vaccine doses. Finally, these results might not be generalizable to other target groups or to countries with different viral circulation and vaccination strategies.
Implications for Public Health Practice
Interim VE estimates from the REVELAC-i Network suggest that influenza vaccines are effective in preventing approximately one third of influenza-related hospitalizations among groups prioritized for vaccination. Although Southern Hemisphere influenza VE is not necessarily predictive of Northern Hemisphere VE, it can help the Northern Hemisphere plan contingencies for vaccination demand and use. These data suggest that influenza vaccine demand was still low post–COVID-19 but that vaccination prevented approximately one third of influenza-associated hospitalizations among groups at high risk for influenza-associated complications. These findings support CDC and WHO’s recommendation that all eligible persons aged ≥6 months should receive influenza vaccination (5,8). If similar influenza viruses continue to predominate during Northern Hemisphere influenza season and the updated A/Thailand/8/2022 (H3N2)–like antigen provides similar protection against circulating influenza A(H3N2) viruses, health authorities might anticipate similar levels of protection. Nonpharmaceutical measures, such as hand washing and mask use, and early antiviral treatment can complement vaccination for stronger protection against influenza illness and its complications.
Acknowledgments
Michael Jhung, Marie Kirby, John Steele, Angie Trujillo, Influenza Division, National Center for Immunization and Respiratory Diseases, CDC; Florencia Bruggesser (Argentina), Claudio Canales (Chile), Lely Guzmán, Agustina Iovanne, Wilmer Marquiño, Patricia Marques (Brazil), Haydee Padilla, Grisel Rodríguez, Solange Santillana, Noelia Speranza (Uruguay), Neris Villalobos (Paraguay), Pan American Health Organization REVELAC-i Network country focal points.
REVELAC-i Network
Estefania Benedetti, INEI-ANLIS–Dr. Carlos G. Malbrán, Buenos Aires, Argentina; Andrea Pontoriero, INEI-ANLIS–Dr. Carlos G. Malbrán, Buenos Aires, Argentina; Maria del Valle Juarez, Ministry of Health, Buenos Aires, Argentina; Nathalia Katz, Ministry of Health, Buenos Aires, Argentina; Maria Paz Rojas Mena, Ministry of Health, Buenos Aires, Argentina; Carla Jimena Voto, Ministry of Health, Buenos Aires, Argentina; Walquiria Aparecida Ferreira da Almeida, Ministry of Health, Brasília, Brazil; Daiana Araújo da Silva, Ministry of Health, Brasília, Brazil; Francisco José de Paula Júnior, Ministry of Health, Brasília, Brazil; Felipe Cotrim de Carvalho, Ministry of Health, Brasília, Brazil; Ana Catarina de Melo Araujo, Ministry of Health, Brasília, Brazil; Greice Madeleine Ikeda do Carmo, Ministry of Health, Brasília, Brazil; Carlos Edson Hott, Ministry of Health, Brasília, Brazil; Miriam Teresinha Furlam Prando Livorati, Ministry of Health, Brasília, Brazil; Marcela Avendaño, Ministry of Health, Santiago, Chile; María Fernanda Olivares Barraza, Ministry of Health, Santiago, Chile; Patricia Bustos, Ministry of Health, Santiago, Chile; Paula Rodríguez Ferrari, Ministry of Health, Santiago, Chile; Natalia Vergara Mallegas, Ministry of Health, Santiago, Chile; Rodrigo Fasce Pineda, Ministry of Health, Santiago, Chile; Silvia Battaglia, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Marta Von Horoch, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Chavely Domínguez, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Maria José Ortega, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Elena Penayo, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Cynthia Vázquez, Ministry of Public Health and Social Welfare, Asunción, Paraguay; Hector Chiparelli, Ministry of Public Health, Montevideo, Uruguay; Natalia Goñi, Ministry of Public Health, Montevideo, Uruguay; Karina Griot, Ministry of Public Health, Montevideo, Uruguay; Jose Eduardo Silvera, Ministry of Public Health, Montevideo, Uruguay; Daiana Tritten, Ministry of Public Health, Montevideo, Uruguay; Steven Tapia Villacís, Ministry of Public Health, Montevideo, Uruguay
GISAID Laboratories
Argentina: INEI-ANLIS–Dr. Carlos G. Malbrán. Brazil: CDC, Evandro Chagas Institute, Ezequiel Dias Foundation (FUNED), FUNED MG, Instituto Adolfo Lutz – National Influenza Center, Instituto Butantan, Instituto de Biotecnologia – ibtec, Instituto Oswaldo Cruz, Instituto Oswaldo Cruz FIOCRUZ – Laboratory of Respiratory Viruses and Measles (LVRS), Laboratório Central de Saúde Pública (LACEN RJ), Laboratório Central de Saúde Pública de Alagoas, LACEN-AL, Laboratório Central de Saúde Pública de São Paulo – Instituto Adolfo Lutz, Laboratório Central de Saúde Pública do Distrito Federal, Laboratório Central de Saúde Pública Professor Gonçalo Moniz, LACEN-BA, Laboratório Central do Estado do Paraná – LACEN/PR, Laboratorio Central do Estado do Rio de Janeiro – LACEN/RJ – Noel Nutels, Laboratorio Professor Eleuterio LACEN SC, LACEN/CE – Laboratório Central de Saúde Pública do Ceará, LACEN/ES – Laboratório Central de Saúde Pública do Espírito Santo, LACEN/SE – Laboratório Central de Saúde Pública de Sergipe – Instituto Parreira Horta, Molecular Biology/Microbiology Research Laboratory, Universidade Federal do Paraná, Oswaldo Cruz Foundation São Paulo State University – Biotechnology Institute, University of São Paulo. Chile: CDC, Instituto de Salud Pública de Chile, Instituto de Salud Pública de Chile. Paraguay: Laboratorio Central de Salud Publica. Uruguay: Departamento de Laboratorio de Salud Pública.
Corresponding author: Erica E. Zeno, ezeno@cdc.gov.
1Influenza Division, National Center for Immunization and Respiratory Diseases, CDC; 2Epidemic Intelligence Service, CDC; 3Pan American Health Organization, Washington, DC; 4School of Nursing and Health Professions, University of San Francisco, San Francisco, California; 5Área de Vigilancia, Dirección de Epidemiología, Ministerio de Salud, Buenos Aires, Argentina; 6Dirección de Control de Enfermedades Inmunoprevenibles, Ministerio de Salud, Buenos Aires, Argentina; 7Laboratorio Nacional de Referencia INEI-ANLIS–Dr. Carlos G. Malbrán, Buenos Aires, Argentina; 8Ministry of Health, Brasília, Brazil; 9Departamento de Epidemiologia – Ministerio de Salud, Santiago, Chile; 10Dirección General de Vigilancia de la Salud, Ministerio de Salud Pública y Bienestar Social, Asunción, Paraguay; 11Programa Ampliado de Inmunizaciones, Ministerio de Salud Pública y Bienestar Social, Asunción, Paraguay; 12Laboratorio Central de Salud Pública, Ministerio de Salud Pública y Bienestar Social, Asunción, Paraguay; 13Departamento de Vigilancia en Salud, Control de Infecciones Hospitalarias, Ministerio de Salud Pública, Montevideo, Uruguay; 14Departamento de Laboratorios de Salud Pública, Ministerio de Salud Pública, Montevideo, Uruguay.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Annette Regan reports receipt of travel support from the International Society for Influenza and Other Respiratory Virus Diseases to attend and present at the 2024 meeting of Options for Control of Influenza, in Brisbane, Australia and from the International Neonatal and Maternal Immunization Symposium to present at the 2024 meeting in San Jose, Costa Rica, and participation on a data safety monitoring board for Moderna’s candidate mRNA RSV vaccine for pregnant women. No other potential conflicts of interest were disclosed.
* These authors contributed equally to this report.
** Young children were defined as those aged 6 months–2 years (Argentina), 6 months–3 years (Paraguay), 6 months–5 years (Chile and Uruguay), and 6 months–6 years (Brazil). Older adults were defined as those aged ≥60 years (Brazil and Paraguay) and those aged ≥65 years (Argentina, Chile, and Uruguay). The preexisting conditions tracked by REVELAC-i are asthma, cancer, hypertension, diabetes, cardiovascular disease, respiratory disease (excluding asthma), obesity, and immunocompromise.
†† Influenza vaccination campaign start dates were Argentina: March 21; Brazil (except for the Northern Region): March 25, Chile: March 13, Paraguay: April 3, and Uruguay: April 24.
¶¶ VE was estimated using multivariable logistic regression as (1 − adjusted odds ratio) × 100%.
*** 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
§§§ https://joss.theoj.org/papers/10.21105/joss.03773
References
- Palekar RS, Rolfes MA, Arriola CS, et al. Burden of influenza-associated respiratory hospitalizations in the Americas, 2010–2015. PLoS One 2019;14:e0221479. https://doi.org/10.1371/journal.pone.0221479 PMID:31490961
- Iuliano AD, Roguski KM, Chang HH, et al.; Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018;391:1285–300. https://doi.org/10.1016/S0140-6736(17)33293-2 PMID:29248255
- Nogareda F, Gharpure R, Contreras M, et al. Seasonal influenza vaccination in the Americas: progress and challenges during the COVID-19 pandemic. Vaccine 2023;41:4554–60. https://doi.org/10.1016/j.vaccine.2023.06.024 PMID:37328348
- Doll MK, Pettigrew SM, Ma J, Verma A. Effects of confounding bias in coronavirus disease 2019 (COVID-19) and influenza vaccine effectiveness test-negative designs due to correlated influenza and COVID-19 vaccination behaviors. Clin Infect Dis 2022;75:e564–71. https://doi.org/10.1093/cid/ciac234 PMID:35325923
- World Health Organization. Vaccines against influenza: WHO position paper – May 2022. Geneva, Switzerland: World Health Organization; 2022;97:185–208. https://www.who.int/publications/i/item/who-wer9719
- Arriola CS, El Omeiri N, Azziz-Baumgartner E, et al.; Influenza vaccine effectiveness against hospitalizations in children and older adults—data from South America, 2013–2017. a test negative design. Vaccine X 2019;3:100047. https://doi.org/10.1093/cid/ciz411 PMID:31102401
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2024–2025 Northern Hemisphere influenza season. Geneva, Switzerland: World Health Organization; 2024. https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season
- Grohskopf LA, Blanton LH, Ferdinands JM, Chung JR, Broder KR, Talbot HK. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2023–24 influenza season. MMWR Recomm Rep 2023;72:1–25. https://doi.org/10.15585/mmwr.rr7202a1
Abbreviations: REVELAC-i = La Red para la Evaluación de Vacunas en Latino América y el Caribe – influenza; SARI = severe acute respiratory infection.
* Argentina, Brazil, Chile, Paraguay, and Uruguay.
† Patients who received ≥1 dose of the 2024 season influenza vaccine ≥14 days before symptom onset were considered vaccinated; patients who did not receive any influenza vaccine during the 2024 season by the time of symptom onset were considered unvaccinated. Patients vaccinated 0–13 days before symptom onset or who received positive SARS-CoV-2 reverse transcription–polymerase chain reaction test results were excluded from the evaluation to avoid the risk of confounding.
§ A Pearson’s chi-square test was used to ascertain whether there were differences in the numbers of persons who were vaccinated and unvaccinated or who received positive and negative influenza test results.
¶ Target groups are included as mutually exclusive groups of patients considered to be at high risk for severe outcomes associated with influenza infection. Young children were defined as those aged 6 months–2 years (Argentina), 6 months–3 years (Paraguay), 6 months–5 years (Chile and Uruguay), and 6 months–6 years (Brazil). Older adults were defined as those aged ≥60 years (Brazil and Paraguay) and aged ≥65 years (Argentina, Chile, and Uruguay). The preexisting conditions tracked by REVELAC-i are asthma, cancer, hypertension, diabetes, cardiovascular disease, respiratory disease (excluding asthma), obesity, and immunocompromise.
FIGURE. Patients hospitalized with severe acute respiratory infection who received positive influenza virus test results,* by epidemiologic week,† (N = 11,751) — REVELAC-i Network, five South American countries,§ March–July 2024
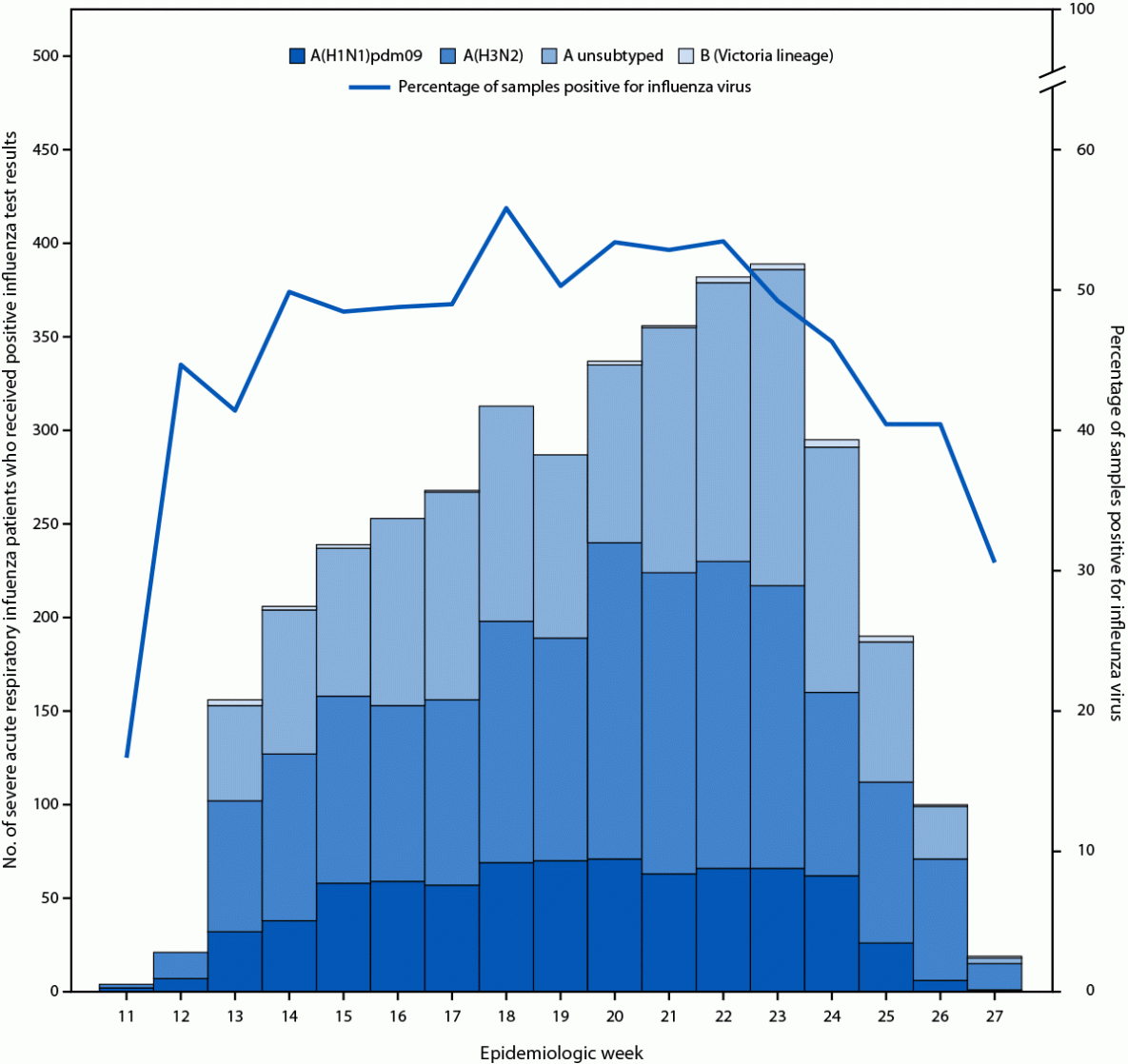
* By reverse transcription–polymerase chain reaction testing at national reference laboratories.
† Epidemiologic week 11 began on March 10, 2024; epidemiologic week 27 ended on July 6, 2024.
§ Argentina, Brazil, Chile, Paraguay, and Uruguay.
Abbreviation: NC = not calculated.
* Argentina, Brazil, Chile, Paraguay, and Uruguay.
† Reverse transcription polymerase–chain reaction testing for influenza was conducted at national reference laboratories.
§ Vaccine effectiveness estimated from logistic regression model adjusting for participating country, sex, age in years (fit as cubic spline), week of onset of symptoms (fit as cubic spline), and presence of at least one comorbidity.
¶ Young children were defined as those aged 6 months–2 years (Argentina), 6 months–3 years (Paraguay), 6 months–5 years (Chile and Uruguay), and 6 months–6 years (Brazil). Older adults were defined as those aged ≥60 years (Brazil and Paraguay) and aged ≥65 years (Argentina, Chile, and Uruguay).
** Percentage was NC when fewer than five patients were in each of the categories.
Suggested citation for this article: Zeno EE, Nogareda F, Regan A, et al. Interim Effectiveness Estimates of 2024 Southern Hemisphere Influenza Vaccines in Preventing Influenza-Associated Hospitalization — REVELAC-i Network, Five South American Countries, March–July 2024. MMWR Morb Mortal Wkly Rep 2024;73:861–868. DOI: http://dx.doi.org/10.15585/mmwr.mm7339a1.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.