Notes from the Field: House-to-House Campaign Administration of Inactivated Poliovirus Vaccine — Sokoto State, Nigeria, November 2022
Weekly / November 24, 2023 / 72(47);1290–1291
Oladayo Biya, MD1; Jibrin Idris Manu, MSc, MPH2; Joseph C. Forbi, PhD1; Gatei wa Nganda, MD1; Hadley Ikwe, MBBS1; Adamu Sule, MBBS1; Aboyowa Edukugho, DVM2; Abba Shehu, MBBS2; Nurudeen Aliyu, MBBS2; Nyampa David Barau, MPH2; Eric Wiesen, DrPH1; Roland W. Sutter, MD1 (View author affiliations)
View suggested citationAltmetric:
After the 2015 documentation of global eradication of wild poliovirus type 2,* Sabin type 2 oral poliovirus vaccine (OPV) was withdrawn from routine immunization (RI) in all OPV-using countries in 2016, in a global synchronized switch from trivalent OPV (containing vaccine virus serotypes 1, 2, and 3) to bivalent OPV (containing serotypes 1 and 3), to reduce the rare risks for type 2 vaccine-associated paralytic poliomyelitis. Concurrently, the Global Polio Eradication Initiative (GPEI) recommended that all OPV-using countries introduce ≥1 dose of inactivated poliovirus vaccine (IPV) into RI programs; IPV protects against paralysis caused by all three serotypes but cannot be transmitted from person to person or cause paralysis. Use of OPV, especially in areas with low vaccination coverage, is associated with low risk of emergence of vaccine-derived polioviruses (VDPVs). As susceptible persons in new birth cohorts accumulated after withdrawal of OPV type 2, population immunity against infection with serotype 2 declined (1), facilitating the emergence of circulating VDPV type 2 (cVDPV2). During the previous 7 years, cVDPV2 outbreaks required response supplementary immunization activities (SIAs) with monovalent type 2 OPV (mOPV2); however, if SIAs were not of sufficiently high quality and did not achieve high enough coverage, new emergences of cVDPV2 occurred.
Background
Routine administration of 1 dose of IPV at age 14 weeks, which was recommended by GPEI following the switch, provides protection against paralysis caused by all three poliovirus serotypes to approximately 60% of recipients (2); however, 1-dose RI IPV coverage is low in many countries. A substantial number of subnational jurisdictions in Nigeria reported RI IPV coverage <50%, including many in the northern part of the country, based on a combined National Immunization Coverage Survey and Multiple Indicator Cluster Survey conducted in 2021† to assess vaccination coverage and various aspects of children’s health and education.
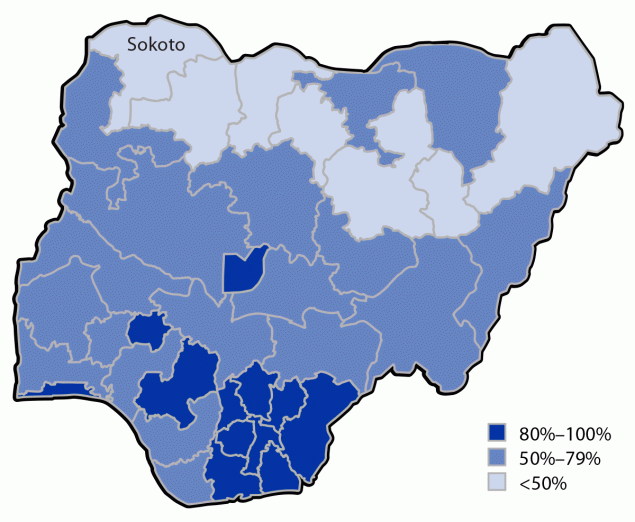
Controlling cVDPV2 outbreaks requires conducting multiple SIAs. In 2021, novel OPV2 (nOPV2), a more genetically stable version of OPV2 that is less likely to revert to neurovirulence in settings of low population immunity, replaced mOPV2 (3). However, if these campaigns do not reach a high proportion of resident children, cVDPV2 circulation could continue. In Nigeria’s northwest Sokoto State, outbreak transmission continued even after eight nOPV2 SIAs conducted since March 2021 (National Primary Health Care Development Agency, Polio Expert Review Committee meeting, Abuja, Nigeria, unpublished data, 2023). Because Sokoto reported 27% RI IPV coverage in 2021 (Figure), a campaign to increase IPV coverage was planned. To conserve limited IPV resources, a 2-dose fractional-dose IPV (fIPV) series, which consists of an intradermal injection of one fifth of a full intramuscular IPV dose, can be administered instead of a singular intramuscular dose (4). The 2 doses are administered at an interval of ≥4 weeks. A large SIA with fIPV administered at fixed-post immunization sites has been implemented in Pakistan, with coverage of 85% (5).
fIPV Vaccination Campaign and Postcampaign Coverage Survey
To evaluate whether fIPV could be administered in a house-to-house campaign using a needle-free jet injection device (Tropis, Pharmajet§), a pilot project was conducted in Sarkin Adar Gidan Igwai, a ward (subdistrict) of Sokoto State. One fIPV dose was added to an already planned nOPV2 SIA in November 2022, targeting children aged 3–59 months. Nurses were trained to use the devices before they were deployed with nOPV2 vaccination teams. The fIPV dose was withdrawn from a multidose vial into a cartridge in each home. Field evaluation conducted at the time of fIPV vaccination documented that a majority of parents (94%) and health staff members (93%) preferred needle-free injections over the customary needle and syringe administration. This activity was reviewed by CDC, deemed not research, and was conducted consistent with applicable federal law and CDC policy.¶
To assess postcampaign fIPV coverage, a survey was conducted using the World Health Organization modified cluster survey technique to sample 210 children aged 3–59 months from 30 settlements in the pilot ward. The coverage survey indicated that 87% of children in the target age group had received fIPV during the campaign.
Preliminary Conclusions and Actions
This pilot study demonstrated that administering an injectable vaccine in a house-to-house campaign with needle-free jet injector devices is feasible and can achieve high coverage. Intensification of RI, including increasing immunization sessions, provision of supportive supervision, and ensuring vaccine availability, will be needed to complete vaccination of children in the pilot ward with the second fIPV dose. Additional pilot studies targeting larger populations should be conducted before this approach can be applied in other low-IPV coverage areas.
Acknowledgments
Tabawa Abubakar, Zaitun Ibrahim Saleh, National Primary Health Care Development Agency, Abuja, Nigeria.
Corresponding author: Oladayo Biya, obiya@cdc.gov.
1Global Immunization Division, Center for Global Health, CDC; 2African Field Epidemiology Network, Abuja, Nigeria.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Roland W. Sutter reports ownership of 5,000 shares of Pharmajet stock (no value declared). No other potential conflicts of interest were disclosed.
* https://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/
† https://www.unicef.org/nigeria/media/6316/file/2021%20MICS%20full%20report%20.pdf
§ https://pharmajet.com/tropis-id/
¶ 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
References
- Cooper LV, Bandyopadhyay AS, Gumede N, et al. Risk factors for the spread of vaccine-derived type 2 polioviruses after global withdrawal of trivalent oral poliovirus vaccine and the effects of outbreak responses with monovalent vaccine: a retrospective analysis of surveillance data for 51 countries in Africa. Lancet Infect Dis 2022;22:284–94. https://doi.org/10.1016/S1473-3099(21)00453-9 PMID:34648733
- Fox JP. Modes of action of poliovirus vaccines and relation to resulting immunity. Rev Infect Dis 1984;6(Suppl 2):S352–5. https://doi.org/10.1093/clinids/6.Supplement_2.S352 PMID:6740072
- Voorman A, Lyons H, Shuab F, et al. Evaluation of novel oral polio vaccine type 2 SIA impact in a large outbreak of circulating vaccine-derived poliovirus in Nigeria. J Infect Dis 2023;jiad222. https://doi.org/10.1093/infdis/jiad222 PMID:37357964
- Anand A, Zaman K, Estívariz CF, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 2015;33:6816–22. https://doi.org/10.1016/j.vaccine.2015.09.039 PMID:26476367
- Bullo UF, Mehraj J, Raza SM, et al. An experience of mass administration of fractional dose inactivated polio vaccine through intradermal needle-free injectors in Karachi, Sindh, Pakistan. BMC Public Health 2021;21:44. https://doi.org/10.1186/s12889-020-10041-8 PMID:33407294
 FIGURE. Inactivated poliovirus vaccine 1-dose coverage, by state — National Immunization Coverage Survey and Multiple Indicator Cluster Survey, Nigeria, 2021
FIGURE. Inactivated poliovirus vaccine 1-dose coverage, by state — National Immunization Coverage Survey and Multiple Indicator Cluster Survey, Nigeria, 2021
Suggested citation for this article: Biya O, Manu JI, Forbi JC, et al. Notes from the Field: House-to-House Campaign Administration of Inactivated Poliovirus Vaccine — Sokoto State, Nigeria, November 2022. MMWR Morb Mortal Wkly Rep 2023;72:1290–1291. DOI: http://dx.doi.org/10.15585/mmwr.mm7247a3.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.