Rapid Analysis of Drugs: A Pilot Surveillance System To Detect Changes in the Illicit Drug Supply To Guide Timely Harm Reduction Responses — Eight Syringe Services Programs, Maryland, November 2021–August 2022
Weekly / April 28, 2023 / 72(17);458–462
Erin Russell, MPH1; Edward Sisco, PhD2; Allison Thomson, MPH3; Jasmine Lopes, MPH4; Margaret Rybak, MPH4; Malik Burnett, MD1; Dana Heilman, MPH5; Meghan G. Appley, PhD2; R. Matt Gladden, PhD6 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Illicitly manufactured fentanyl was involved in 84% of 2,912 drug overdose deaths in Maryland during July 2020–June 2021.
What is added by this report?
Among 364 samples from drug paraphernalia collected at eight syringe services programs during November 2021–August 2022 that tested positive for fentanyl or fentanyl analogs, 80% also contained xylazine (an animal sedative). Heroin was rarely detected. Results were available within 48 hours. Sample test results did not always differ from participant expectations and were used to enhance harm reduction efforts.
What are the implications for public health practice?
Rapid analysis of drug paraphernalia can provide timely data on changing illicit drug markets that can be used to mitigate the harms of drug use more effectively.
Altmetric:
A record number of 2,912 drug overdose deaths occurred in Maryland during the 12-month period July 1, 2020–June 30, 2021. Illicitly manufactured fentanyl, fentanyl analogs, or both* were involved in 84% of these deaths.† Timely identification of illicit drug market changes (e.g., fentanyl rapidly replacing heroin) could improve the public health response, specifically communications about risks for novel psychoactive substances. During November 19, 2021–August 31, 2022, the National Institute of Standards and Technology (NIST)§ tested 496 deidentified drug paraphernalia samples that staff members collected at eight Maryland syringe services programs (SSPs), also known as needle exchange programs,¶ in partnership with the Maryland Department of Health Center for Harm Reduction Services (CHRS).** All test results were available within 48 hours. Among the 496 paraphernalia samples collected, 367 (74.0%) tested positive for an opioid, and 364 (99.2%) of these samples contained fentanyl or fentanyl analogs. Approximately four fifths of fentanyl-positive samples also tested positive for the veterinary medicine xylazine, a sedative that when combined with opioids might increase the potential for fatal respiratory depression and soft tissue infections when injected (1). For 248 of the 496 samples, SSP participants also completed a questionnaire about the drugs they had intended to purchase. Among the 212 participants who had intended to buy an opioid, 87.7% were exposed to fentanyl, fentanyl analogs, or both, and 85.8% were unknowingly exposed to xylazine. Results improved awareness of fentanyl and xylazine among SSP staff members and galvanized efforts to enhance SSPs’ wound care services for participants experiencing soft tissue injuries possibly associated with injecting xylazine. Rapid analysis of drug paraphernalia can provide timely data on changing illicit drug markets that can be used to mitigate the harms of drug use more effectively.
In June 2021, CHRS, which oversees SSPs in Maryland, and NIST implemented the Rapid Analysis of Drugs (RAD) program to address the need for rapid, comprehensive, and reliable identification of illicit drugs. Twelve pilot sites were selected based on each site’s capacity and proximity to drug trafficking routes identified by law enforcement partners. In August 2021, eight of the 12 SSPs that were contacted agreed to participate.†† Staff members attended a virtual training covering RAD’s legal context and processes, including how to collect samples as safely as possible from used paraphernalia. Program staff members then sampled multiple types of drug paraphernalia, excluding syringes.
RAD involves a four-step process. First, wearing gloves, SSP staff members wipe or swab used drug paraphernalia received from registered SSP participants. Each individual wipe or swab is then placed into a small paper envelope that is collected in a larger mailing envelope (2). Program staff members administered a deidentified questionnaire simultaneously with paraphernalia sample collection and linked the questionnaire and sample with a unique barcode number.§§ Second, samples are mailed to NIST in accordance with U.S. Postal Service regulations. Third, samples are extracted and analyzed using direct analysis in real time mass spectrometry (DART-MS), a rapid ambient ionization mass spectrometry screening technique capable of analyzing a sample in seconds and detecting more than 1,100 drugs, cutting agents, and related substances¶¶ (3). Fourth, within 48 hours, NIST reports substances identified in each sample to CHRS and SSPs.*** SSPs are then responsible for sharing individual results back to the participant who submitted the sample. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.†††
During November 19, 2021–August 31, 2022, staff members from eight SSPs asked program participants for permission to collect a sample from their used paraphernalia for drug testing and to complete a questionnaire about the drugs they had intended to purchase. A total of 496 paraphernalia samples were collected. For 248 (50.0%) of these samples, the program participant completed the questionnaire. No overdoses occurred on-site during sampling. The five most common types of paraphernalia tested, accounting for 95.7% of samples, were plastic bags (54.8%), cookers (16.3%), capsules (11.7%), vials (6.9%), and pipes or straws (6.0%). Among the 496 samples, one or more opioids were detected in 367 (74.0%) and cocaine in 77 (15.5%); none of the screened drugs were detected in 26 (5.2%) samples. Among the 367 opioid-positive samples, 363 (98.9%) contained fentanyl, 23 (6.3%) fluorofentanyl, and six (1.6%) fentanyl carbamate. One sample contained fluorofentanyl only; all other fentanyl analogs (e.g., fluorofentanyl and fentanyl carbamate) were also detected with fentanyl. Nonfentanyl opioids were detected infrequently: heroin (1.9%), tramadol (1.6%), methadone (0.5%), and protonitazene (0.3%). Among samples positive for fentanyl or a fentanyl analog (364), 84.4% had at least one other stimulant, sedative, or benzodiazepine detected: 293 (80.5%) had xylazine, 23 (6.3%) cocaine, 10 (2.7%) synthetic cathinones, six (1.6%) benzodiazepines, and three (0.8%) amphetamines (Figure).
Questionnaires were submitted for 248 (50.0%) samples.§§§ Among 212 respondents who reported opioid purchases,¶¶¶ 50.9% intended to purchase both heroin and fentanyl, or “fentanyl and/or heroin,”**** 46.7% sought fentanyl alone, and 2.4% sought heroin alone (Table). Eighty-one percent of samples matched the participant’s intentions but contained one or more additional substances, 13.2% did not include the substance the participant intended to purchase, and 5.7% matched participant intentions without other substances present. When the participant reported intent to buy heroin, no sample tested positive for heroin, and 1.9% of samples tested positive for heroin when the participant reported buying “fentanyl and heroin.” When participants reported intent to buy “fentanyl” or “fentanyl and heroin,” 97.0% and 79.6% of the samples, respectively, tested positive for fentanyl. When participants intended to buy “fentanyl” or “fentanyl and/or heroin,” xylazine was detected in 90.9% and 84.3% of samples, respectively. The questionnaire did not indicate xylazine in the list of drugs that participants might have intended to purchase; if they wanted to purchase xylazine, they would have needed to write it in an “other” drug category, and none of the participants did.
Discussion
RAD supported Maryland’s public health response to overdose deaths by quickly identifying the broad adulteration of fentanyl with xylazine and documenting the dominance of fentanyl (including samples mixed with fluorofentanyl) (4) and absence of heroin. Because of the success of the eight RAD pilot sites, CHRS expanded RAD to all Maryland SSPs during 2022; as of April 2023, 14 programs are participating.
Xylazine’s pervasiveness as an adulterant was unexpected by CHRS, program staff members, and participants, but aligned with observational evidence about an increase in injection-related wounds observed in other reports on xylazine (1). Wounds might appear outside the area of injection and might also occur when xylazine is smoked or snorted. Documenting the widespread adulteration of fentanyl with xylazine facilitated stronger communication about the risk for injection-related wounds with participants. CHRS is investing in wound care training and certification for nurses working in harm-reduction settings, creating standards of care for wound treatment in low-resourced settings, and pursuing quantitative data analysis opportunities to corroborate the relationship between statewide incidence of skin and soft tissue infections and the drug supply revealed through RAD.
Co-use of xylazine with fentanyl might increase the chance of fatal overdose.†††† The effects of xylazine are not reversed by naloxone and might require medical care; however, naloxone does reverse the effects of fentanyl and other opioids even when co-used with xylazine and should be administered for any suspected overdose (1,5). In response to these findings, CHRS and SPPs updated overdose response training to include managing xylazine-involved overdoses.
More than one half of questionnaire respondents intending to purchase opioids thought heroin might be present with fentanyl in the drugs they purchased, whereas heroin was present in fewer than 2% of samples. Because fentanyl is fast-acting and potent, it might lead to rapid onset of overdose (6). This finding reinforces the continued importance of fentanyl-specific overdose education efforts in Maryland. This instruction includes injecting slowly, not using drugs when alone, using fentanyl test strips, carrying naloxone, and seeking and accepting medical attention for an overdose.§§§§
The findings in this report are subject to at least three limitations. First, drug paraphernalia were conveniently sampled from eight SSPs that primarily serve persons who use opioids and inject drugs; results are not necessarily generalizable to all persons using drugs or in other geographic regions. Second, syringes were not included in RAD because of sampling safety. Finally, DART-MS analysis is not quantitative, and substance purity was not measured.
Timely data about the illicit drug supply have been limited, retrospective, and often anecdotal. This report provides critical information on fentanyl and xylazine exposures among a population at high risk for overdose and related harm. In some areas, fentanyl is adulterated with emerging substances such as xylazine (7). Also, heroin-involved overdoses have substantially declined in some places as fentanyl-involved overdoses have become more dominant.¶¶¶¶ RAD can provide timely data on the rapid increase of common illicit drugs (e.g., fentanyl) as well as influx of emerging substances (e.g., xylazine) that can help harm reduction programs mitigate the health impact more effectively. This in turn might strengthen participants’ trust in SSPs, which might increase participants’ likelihood of seeking treatment and reducing their drug use (8). Providing persons who use drugs with timely data on the drugs they are using versus what they intended to use might also reduce public health harms (9,10).
Corresponding author: Erin Russell, erin.russell@maryland.gov.
1Center for Harm Reduction Services, Maryland Department of Health; 2National Institute of Standards and Technology, U.S. Department of Commerce, Gaithersburg, Maryland; 3Division of HIV Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, CDC; 4CDC Foundation, Atlanta, Georgia; 5National Governor’s Association, Washington, DC; 6Division of Overdose Prevention, National Center for Injury Prevention and Control, CDC.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Margaret Rybak reports contract employment with the CDC Foundation under the Overdose Data to Action Grant. Jasmine Lopes reports contract employment with the CDC Foundation under the Overdose Data to Action Grant. No other potential conflicts of interest were disclosed.
* Fentanyl analogs, also known as fentanyl-related substances, vary in potency and are synthetic opioids similar in chemical structure to fentanyl but modified to produce distinct substances. Fentanyl analogs include acetyl fentanyl, acryl fentanyl, butyryl fentanyl, despropionyl fentanyl, and 4-fluorofentanyl.
† https://beforeitstoolate.maryland.gov/oocc-data-dashboard
¶ https://www.cdc.gov/ssp/index.html
** https://health.maryland.gov/phpa/Pages/accessharmreduction.aspx
†† The four SSPs that did not participate cited political pushback and insufficient capacity to implement RAD. Capacity issues included inadequate staffing and safety concerns about handling the paraphernalia on-site because SSPs typically only collect syringes for off-site disposal using biohazard containers that require no contact with staff members. As of January 10, 2023, Maryland had 22 active SSPs.
§§ Responses were documented into a webform accessible by CHRS staff members.
¶¶ Data are interpreted using libraries, specifically the NIST and National Institute of Justice DART-MS Data Interpretation Tool and NIST DART-MS Forensics Database (version 1.5; Firefly), enabling identification of more than 1,100 drugs, cutting agents, and related substances (https://data.nist.gov/od/id/mds2-2448). DART-MS cannot differentiate some isomers from one another.
*** NIST shares sample results with CHRS virtually via Google Workspace. CHRS merges the NIST results with the questionnaire responses and makes the deidentified data available to all participating SSPs also using Google Workspace.
††† 5 C.F.R. part 46; 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d), 5 U.S.C. Sect. 552a, 44 U.S.C. Sect. 3501 et seq.
§§§ Questionnaire responses that reported sampled paraphernalia being used more than once were excluded from analysis on intended purchased substance.
¶¶¶ Four instances of intended purchase of opioid pills were excluded from analysis because of inability to ascertain which opioid was the intended purchase.
**** Some participants who selected the response categories “heroin” and “fentanyl” might have been trying to indicate that they did not know if the drug product was solely heroin or fentanyl.
§§§§ https://www.goslow.org; https://www.samhsa.gov/find-help/harm-reduction
¶¶¶¶ https://www.chicagohan.org/documents/14171/234367/2021_MidYr_Opioid_Report_AUg2021.pdf; https://www.hamiltoncountyhealth.org/wp-content/uploads/2021Snapshot_HCARC_20221014-6.pdf; https://www.nyc.gov/assets/doh/downloads/pdf/epi/databrief133.pdf; https://ocme.dc.gov/sites/default/files/dc/sites/ocme/Opioid%20related%20Overdoses%20Deaths%208.18.22%20FINAL.pdf
References
- Alexander RS, Canver BR, Sue KL, Morford KL. Xylazine and overdoses: trends, concerns, and recommendations. Am J Public Health 2022;112:1212–6. https://doi.org/10.2105/AJPH.2022.306881 PMID:35830662
- Sisco E, Robinson EL, Burns A, Mead R. What’s in the bag? Analysis of exterior drug packaging by TD-DART-MS to predict the contents. Forensic Sci Int 2019;304:109939. https://doi.org/10.1016/j.forsciint.2019.109939 PMID:31580981
- Appley MG, Robinson EL, Thomson A, Russell E, Sisco E. An analytical platform for near real-time drug landscape monitoring using paraphernalia residues. ChemRxiv . [Preprint posted online December 16, 2022.] https://doi.org/10.26434/chemrxiv-2022-bd64n
- Bitting J, O’Donnell J, Mattson CL. Notes from the field: overdose deaths involving para-fluorofentanyl—United States, July 2020–June 2021. MMWR Morb Mortal Wkly Rep 2022;71:1239–40. https://doi.org/10.15585/mmwr.mm7139a3 PMID:36173752
- Chhabra N, Mir M, Hua MJ, et al. Notes from the field: xylazine-related deaths—Cook County, Illinois, 2017–2021. MMWR Morb Mortal Wkly Rep 2022;71:503–4. https://doi.org/10.15585/mmwr.mm7113a3 PMID:35358161
- Somerville NJ, O’Donnell J, Gladden RM, et al. Characteristics of fentanyl overdose—Massachusetts, 2014–2016. MMWR Morb Mortal Wkly Rep 2017;66:382–6. https://doi.org/10.15585/mmwr.mm6614a2 PMID:28406883
- Friedman J, Montero F, Bourgois P, et al. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend 2022;233:109380. https://doi.org/10.1016/j.drugalcdep.2022.109380 PMID:35247724
- Caroll J, Green T, Noonan R. Evidence-based strategies for preventing opioid overdose: what’s working in the United States. Atlanta, GA: US Department of Health and Human Services, CDC; 2018. https://www.cdc.gov/drugoverdose/pdf/pubs/2018-evidence-based-strategies.pdf
- Measham FC. Drug safety testing, disposals and dealing in an English field: exploring the operational and behavioural outcomes of the UK’s first onsite ‘drug checking’ service. Int J Drug Policy 2019;67:102–7. https://doi.org/10.1016/j.drugpo.2018.11.001 PMID:30541674
- Measham F, Turnbull G. Intentions, actions and outcomes: a follow up survey on harm reduction practices after using an English festival drug checking service. Int J Drug Policy 2021;95:103270. https://doi.org/10.1016/j.drugpo.2021.103270 PMID:33972157
 FIGURE. Samples tested (N = 496) and found to contain selected substances* and number of instances the selected substance was found in combination with fentanyl — eight syringe services programs, Maryland, November 2021–August 2022
FIGURE. Samples tested (N = 496) and found to contain selected substances* and number of instances the selected substance was found in combination with fentanyl — eight syringe services programs, Maryland, November 2021–August 2022
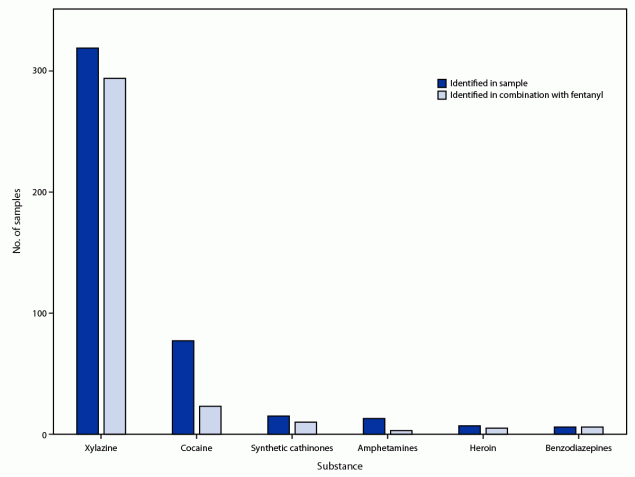
* Samples were analyzed using direct analysis in real time mass spectrometry (DART-MS).
Abbreviation: DART-MS = direct analysis in real time mass spectrometry.
* Identified by DART-MS.
† Tramadol is the only opioid other than fentanyl, fentanyl analogs, and heroin that was detected in samples with survey responses.
§ Other substances include anabolic steroids, anticonvulsants, benzodiazepines, and delta-9-tetrahydrocannabinol.
Suggested citation for this article: Russell E, Sisco E, Thomson A, et al. Rapid Analysis of Drugs: A Pilot Surveillance System To Detect Changes in the Illicit Drug Supply To Guide Timely Harm Reduction Responses — Eight Syringe Services Programs, Maryland, November 2021–August 2022. MMWR Morb Mortal Wkly Rep 2023;72:458–462. DOI: http://dx.doi.org/10.15585/mmwr.mm7217a2.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.