Epidemiologic and Clinical Characteristics of Monkeypox Cases — United States, May 17–July 22, 2022
Weekly / August 12, 2022 / 71(32);1018-1022
On August 5, 2022, this report was posted online as an MMWR Early Release.
Please note: This report has been corrected.
David Philpott, MD1,2; Christine M. Hughes, MPH2; Karen A. Alroy, DVM3; Janna L. Kerins, VMD4; Jessica Pavlick, DrPH5; Lenore Asbel, MD6; Addie Crawley, MPH3; Alexandra P. Newman, DVM7; Hillary Spencer, MD1,4; Amanda Feldpausch, DVM5; Kelly Cogswell, MPH8; Kenneth R. Davis, MPH9; Jinlene Chen, MD10; Tiffany Henderson, MPH11; Katherine Murphy, MPH12; Meghan Barnes, MSPH13; Brandi Hopkins, MPH14; Mary-Margaret A. Fill, MD15; Anil T. Mangla, PhD16; Dana Perella, MPH6; Arti Barnes, MD17; Scott Hughes, PhD3; Jayne Griffith, MPH18; Abby L. Berns, MPH19; Lauren Milroy, MPH20; Haley Blake, MPH21; Maria M. Sievers, MPH22; Melissa Marzan-Rodriguez, DrPH23; Marco Tori, MD1,24; Stephanie R. Black, MD4; Erik Kopping, PhD3,25; Irene Ruberto, PhD26; Angela Maxted, DVM, PhD27; Anuj Sharma, MPH5; Kara Tarter, MPH28; Sydney A. Jones, PhD29,30; Brooklyn White, MPH31; Ryan Chatelain, MPH32; Mia Russo; start highlightSarah Gillani16end highlight; Ethan Bornstein, MD1,8; Stephen L. White, PhD9; Shannon A. Johnson, MPH11; Emma Ortega, MPHTM12; Lori Saathoff-Huber, MPH17; Anam Syed, MPH5; Aprielle Wills, MPH3; Bridget J. Anderson, PhD7; Alexandra M. Oster, MD2; Athalia Christie, DrPH2; Jennifer McQuiston, DVM2; Andrea M. McCollum, PhD2; Agam K. Rao, MD2,*; María E. Negrón, DVM, PhD2,*; CDC Multinational Monkeypox Response Team (View author affiliations)
View suggested citationSummary
What is already known about this topic?
A global monkeypox outbreak began in 2022.
What is added by this report?
Among U.S. monkeypox cases with available data, 99% occurred in men, 94% of whom reported recent male-to-male sexual or close intimate contact; racial and ethnic minority groups appear to be disproportionately affected. Clinical presentations differed from typical monkeypox, with fewer persons experiencing prodrome and more experiencing genital rashes.
What are the implications for public health practice?
Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected, for prevention and testing, address equity, and minimize stigma, while maintaining vigilance for transmission in other populations. Clinicians should test persons with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome.
Altmetric:
Monkeypox, a zoonotic infection caused by an orthopoxvirus, is endemic in parts of Africa. On August 4, 2022, the U.S. Department of Health and Human Services declared the U.S. monkeypox outbreak, which began on May 17, to be a public health emergency (1,2). After detection of the first U.S. monkeypox case), CDC and health departments implemented enhanced monkeypox case detection and reporting. Among 2,891 cases reported in the United States through July 22 by 43 states, Puerto Rico, and the District of Columbia (DC), CDC received case report forms for 1,195 (41%) cases by July 27. Among these, 99% of cases were among men; among men with available information, 94% reported male-to-male sexual or close intimate contact during the 3 weeks before symptom onset. Among the 88% of cases with available data, 41% were among non-Hispanic White (White) persons, 28% among Hispanic or Latino (Hispanic) persons, and 26% among non-Hispanic Black or African American (Black) persons. Forty-two percent of persons with monkeypox with available data did not report the typical prodrome as their first symptom, and 46% reported one or more genital lesions during their illness; 41% had HIV infection. Data suggest that widespread community transmission of monkeypox has disproportionately affected gay, bisexual, and other men who have sex with men and racial and ethnic minority groups. Compared with historical reports of monkeypox in areas with endemic disease, currently reported outbreak-associated cases are less likely to have a prodrome and more likely to have genital involvement. CDC and other federal, state, and local agencies have implemented response efforts to expand testing, treatment, and vaccination. Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected, for prevention and testing, while addressing equity, minimizing stigma, and maintaining vigilance for transmission in other populations. Clinicians should test patients with rash consistent with monkeypox,† regardless of whether the rash is disseminated or was preceded by prodrome. Likewise, although most cases to date have occurred among gay, bisexual, and other men who have sex with men, any patient with rash consistent with monkeypox should be considered for testing. CDC is continually evaluating new evidence and tailoring response strategies as information on changing case demographics, clinical characteristics, transmission, and vaccine effectiveness become available.§
On June 3, 2022, CDC released a case report form for health departments to report monkeypox cases. Data collected include possible exposures during the 3 weeks preceding symptom onset, symptoms during the illness course, and distribution of rash, defined as at least one lesion on the skin or mucous membranes. To describe epidemiologic and clinical characteristics, CDC analyzed case report form data for probable or confirmed cases¶ initially reported through July 22, 2022; to allow for reporting delay, data received through July 27 were included. Analyses were restricted to cases for which relevant data were available. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.**
During May 17–July 22, 2022, a total of 2,891 U.S. monkeypox cases were reported by 43 states, Puerto Rico, and DC; the number of reported cases increased rapidly during this time (Figure). Case report forms including, at minimum, age and gender identity were received for 1,195 (41%) cases; these cases are described in this report. Median age was 35 years (IQR = 30–41 years). Nearly all (99%) persons with case report forms available were men (cisgender and transgender) (Table 1). Among 1,054 cases for which race and ethnicity were reported, 41% occurred among White persons, 28% among Hispanic persons, and 26% among Black persons. Based on information available in case report forms, the percentage of cases among Black persons increased from 12% (29 of 248) during May 17–July 2 to 31% (247 of 806) during July 3–22, and the percentage among Hispanic persons decreased from 33% (82 of 248) to 27% (214 of 806) and among White persons from 49% (121 of 248) to 38% (307 of 806).
Among 241 cases (20%) with reported classification by health departments as being travel-associated or locally acquired, 178 (74%) were classified as locally acquired. The percentage of locally acquired cases increased from 51% (33 of 65) during May 17–July 2 to 82% (145 of 175) during July 3–22.
Among 358 (30%) men (cisgender and transgender) with information on recent sexual behaviors and gender of sex partners available, 337 (94%) reported sex or close intimate contact with a man during the 3 weeks before symptom onset; 16 (4%) reported no such contact. Among 291 men who reported information about their male sexual partners during the 3 weeks preceding symptom onset, 80 (27%) reported one partner, 113 (40%) reported two to four partners, 42 (14%) reported five to nine partners, and 56 (19%) reported 10 or more partners. Among 86 men with information reported, 33 (38%) reported group sex, defined as sex with more than two persons, at a festival, group sex event, or sex party.
The most frequently reported signs and symptoms included rash (100%), fever (63%), chills (59%), and lymphadenopathy (59%) (Table 2). Reported rectal symptoms included purulent or bloody stools (21%), rectal pain (22%), and rectal bleeding (10%). Among 291 persons with available information about their first symptoms, 58% reported at least one prodromal symptom††; for the 42% of patients without prodromal symptoms, illness began with a rash.
Rash was most frequently reported on the genitals (46%), arms (40%), face (38%), and legs (37%); among 718 persons with monkeypox who reported body regions with rash, 238 (33%) reported rash in one region, 126 (18%) in two regions, 98 (14%) in three regions, and 256 (36%) in four or more regions. Among 104 persons with information on the number of lesions, 88% of cases involved fewer than 50 lesions.
Among 334 persons with data available on HIV status, 136 (41%) had HIV infection. Among 954 persons with hospitalization data available, 77 (8%) patients were hospitalized because of their illness. No deaths were reported. Among 339 persons with vaccination status available, 48 (14%) reported previous receipt of smallpox vaccine, including 11 (23%) who received 1 of 2 JYNNEOS doses during the current outbreak, 11 (23%) who received pre-exposure prophylaxis at an unknown time before the current outbreak, and 26 (54%) who did not provide information about when vaccine was administered. Among the recently vaccinated persons with monkeypox, at least one experienced symptoms >3 weeks after their first JYNNEOS dose.
Discussion
Current findings indicate that community transmission of monkeypox is widespread and is disproportionately affecting gay, bisexual, and other men who have sex with men; this is consistent with data reported from other countries (3). Public health efforts to slow monkeypox transmission among gay, bisexual, and other men who have sex with men require addressing challenges that include homophobia, stigma, and discrimination. Although the largest proportion of cases have occurred in White persons, Black and Hispanic persons, who represent approximately one third (34%) of the general population (4), accounted for more than one half (54%) of monkeypox cases in persons for whom information on race and ethnicity is available; further, the proportion of cases among Black persons has increased during recent weeks. Ensuring equity in approaches to monkeypox testing, treatment, and prevention is critical, and taking actions to minimize stigma related to monkeypox can reduce barriers to seeking care and prevention. The data presented in this report provide insights into early transmission; however, ongoing surveillance is essential to monitor future transmission trends and assess the impacts among different communities.
These data can guide clinical considerations for evaluating persons for monkeypox. Typically, monkeypox begins with a febrile prodrome, which might include malaise, chills, headache, or lymphadenopathy, followed by a disseminated rash that often includes the palms and soles (5). Although most cases in this report included these features, 42% of persons did not report prodromal symptoms, and 37% did not report fever by the time of interview. Genital rash, although reported in fewer than one half of cases, was common; 36% of persons developed rash in four or more body regions. Other recent reports describe similar clinical characteristics (6,7). Clinicians should be vigilant for patients with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome. Likewise, although most cases to date have occurred among gay, bisexual, and other men who have sex with men, any patient, regardless of sexual or gender identity, with rash consistent with monkeypox should be considered for testing because close physical contact with an infectious person or exposure to contaminated materials such as clothing or bedding can result in transmission.
A substantial proportion of monkeypox cases have been reported among persons with HIV infection, and efforts are underway to characterize monkeypox clinical outcomes among these persons. Recent reports have found that concurrent sexually transmitted infections were common in persons with monkeypox (3,7). Clinicians and health officials implementing monkeypox education, testing, and prevention efforts should also incorporate recommended interventions for other conditions occurring among gay and bisexual men, including HIV infection, sexually transmitted infections, substance use, and viral hepatitis§§ (8).
On May 23, 2022, CDC launched an emergency response for monkeypox. This response includes educating providers and the public, expanding laboratory testing, outlining prevention strategies, and promoting the use of medical countermeasures for treatment and postexposure prophylaxis. CDC is supporting state, tribal, local, and territorial health departments through guidance and technical assistance. Testing capacity was rapidly expanded through CDC’s Laboratory Response Network and commercial laboratories, with national capacity estimates of 80,000 tests per week by July 18.¶¶
Because of long-standing investments in medical countermeasures for potential smallpox events, licensed vaccines and therapeutics for monkeypox are held in the U.S. Department of Health and Human Services Strategic National Stockpile. A national vaccine strategy was developed to equitably expand vaccination in areas experiencing high numbers of monkeypox cases and contacts. Two vaccines are available in the United States.*** As of August 3, more than 1 million doses of JYNNEOS, a nonreplicating, live virus vaccine (https://www.fda.gov/media/131078/download) had been allocated to jurisdictions, and approximately 14,700 courses of oral tecovirimat (TPOXX) had been distributed to jurisdictions and providers.
The findings in this report are subject to at least three limitations. First, this analysis includes only 41% of U.S. monkeypox cases reported through July 22 and might not be representative of all cases. Jurisdictions with high numbers of cases without submitted case report forms were more racially and ethnically diverse according to U.S. Census Bureau data; therefore, persons from racial and ethnic minority groups might be more disproportionately affected than indicated by these data. Second, even on submitted case report forms, data for variables such as timing of vaccination, sexual behaviors, HIV status, reason for hospitalization, and whether cases were travel-associated were frequently missing; data might also not reflect symptoms or outcomes occurring after the interview. Finally, persons with monkeypox who have mild symptoms might be less likely to seek care or initiate testing and could be underrepresented in this analysis.
CDC is continually evaluating new evidence and tailoring response strategies as information on changing case demographics, clinical characteristics, transmission, and vaccine effectiveness become available. Public health efforts should prioritize gay, bisexual, and other men who have sex with men, who are currently disproportionately affected for prevention and testing, address equity, and minimize stigma, while maintaining vigilance for transmission in other populations. Clinicians should test persons with rash consistent with monkeypox, regardless of whether the rash is disseminated or was preceded by prodrome.
Acknowledgments
Monkeypox response teams from state and local health departments in the following jurisdictions: Arizona, Arkansas, Colorado, Connecticut, Delaware, District of Columbia, Georgia, Hawaii, Idaho, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Minnesota, Missouri, Nebraska, Nevada, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, South Carolina, Tennessee, Texas, Wisconsin, Utah, Virginia, Washington, and West Virginia.
CDC Multinational Monkeypox Response Team
Isabel Griffin, CDC; Mohammed Khan, CDC; Yasmin Ogale, CDC; Emily Sims, CDC; R. Ryan Lash, CDC; Jeanette J. Rainey, CDC; Kelly Charniga, CDC; Michelle A. Waltenburg, CDC; Patrick Dawson, CDC; Laura A.S. Quilter, CDC; Julie Rushmore, CDC; Mark R. Stenger, CDC; Rachel E. Kachur, CDC; Florence Whitehill, CDC; Kelly A. Jackson, CDC; Jim Collins, Michigan Department of Health and Human Services; Kimberly Signs, Michigan Department of Health and Human Services; Gillian Richardson, Louisiana Department of Health; Julie Hand, Louisiana Department of Health; Emily Spence-Davizon, Colorado Department of Public Health and Environment; Brandi Steidley, Colorado Department of Public Health and Environment; Matthew Osborne, Massachusetts Department of Public Health; Susan Soliva, Massachusetts Department of Public Health Joanna Shaw-KaiKai Nashville Metro Public Health Department; Sabrina Cook, Nashville Metro Public Health Department; Leslie Ayuk-Takor, DC Department of Health; Christina Willut, DC Department of Health; Alexandria Snively, Indiana Department of Health; Nicholas Lehnertz, Minnesota Department of Health; Daniela N. Quilliam, Rhode Island Department of Health; Miranda Durham, New Mexico Department of Health; Iris R. Cardona-Gerena, Puerto Rico Department of Health; Linda J. Bell, South Carolina Department of Health; Environmental Control; Marina Kuljanin, Maricopa County Department of Health; Suzanne Gibbons-Burgener, Wisconsin Department of Health Services; Ryan Westergaard, Wisconsin Department of Health Services; Lynn E. Sosa, Connecticut Department of Public Health; Monica Beddo, Missouri Department of Health and Senior Services; Matthew Donahue, Nebraska Department of Health and Human Services; Samir Koirala, Nebraska Department of Health and Human Services; Courtney Dewart, Ohio Department of Health, Career Epidemiology Field Officer, CDC; Jade Murray-Thompson, Utah Department of Health and Human Services; Lilian Peake, Virginia Department of Health; Michelle L. Holshue, Washington Department of Health; Atul Kothari, Arkansas Department of Health; Jamie Ahlers, Delaware Department of Health and Social Services; Lauren Usagawa, Hawaii Department of Health; Megan Cahill, Idaho Division of Public Health; Erin Ricketts, North Carolina Department of Health and Human Services; Mike Mannell, Oklahoma State Department of Health; Farah S. Ahmed, Kansas Department of Health and Environment; Bethany Hodge, Kentucky Department for Public Health; Brenton Nesemeier, North Dakota Department of Health; Katherine Guinther, West Virginia Bureau for Public Health; Madhu Anand, New York State Department of Health; Jennifer L. White, New York State Department of Health; Joel A. Ackelsberg, New York City Department of Health and Mental Hygiene; Ellen H. Lee, New York City Department of Health and Mental Hygiene; Devin Raman, Southern Nevada Health District; Carmen Brown, Pennsylvania Department of Health; Nicole Burton, New York City Department of Health and Mental Hygiene; Sarakay Johnson, Metro Public Health Department–Nashville.
Corresponding author: David Philpott, DPhilpott@cdc.gov.
1Epidemic Intelligence Service; 2CDC Monkeypox Response; 3New York City Department of Health and Mental Hygiene, New York, New York; 4Chicago Department of Health, Chicago, Illinois; 5Georgia Department of Health; 6Philadelphia Department of Public Health, Philadelphia, Pennsylvania; 7New York State Department of Health; 8Oregon Department of Health; 9Texas Department of State Health Services; 10Maryland Department of Health; 11Michigan Department of Health and Human Services; 12Louisiana Department of Health; 13Colorado Department of Public Health and Environment; 14Massachusetts Department of Public Health; 15Tennessee Department of Health; 16DC Department of Health; 17Illinois Department of Public Health; 18Minnesota Department of Health; 19Rhode Island Department of Health; 20Indiana State Department of Health; 21Southern Nevada Health District, Las Vegas, Nevada; 22New Mexico Department of Health; 23Puerto Rico Department of Health; 24South Carolina Department of Health and Environmental Control; 25Laboratory Leadership Service, CDC; 26Arizona Department of Health Services; 27Wisconsin Department of Health Services; 28Ohio Department of Health; 29Connecticut Department of Public Health; 30Career Epidemiology Field Officer Training Program, CDC 31Missouri Department of Health and Senior Services; 31Salt Lake County Health Department, Salt Lake City, Utah; 32Pennsylvania Department of Health.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Mary-Margaret A. Fill reports Council of State and Territorial Epidemiologists (CSTE) travel support to attend annual CSTE conference and uncompensated membership on the University of Tennessee’s One Health Initiative board. No other potential conflicts of interest were disclosed.
* These authors contributed equally to this report.
† https://www.cdc.gov/poxvirus/monkeypox/symptoms.html
§ https://www.cdc.gov/poxvirus/monkeypox/index.html
¶ A probable case was defined as illness for which there was no suspicion of other recent orthopoxvirus exposure and one of the following: 1) detection of orthopoxvirus DNA by polymerase chain reaction testing of a clinical specimen, 2) evidence of orthopoxvirus antigen using immunohistochemical staining or visualization by electron microscopy, or 3) demonstration of detectable levels of antiorthopoxvirus immunoglobulin M antibody during the 4–56 days after rash onset. A confirmed case was defined as 1) the presence of Monkeypox virus DNA by polymerase chain reaction testing or Next-Generation sequencing of a clinical specimen or 2) isolation of Monkeypox virus in culture from a clinical specimen.
** 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
†† Prodrome defined as at least one of the following: fever, myalgias, malaise, headaches, lymphadenopathy, or chills occurring as first symptom, not accompanied by a rash.
§§ https://www.cdc.gov/msmhealth/index.htm
*** https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
References
- Diamond D. Monkeypox is a public health emergency. The Washington Post. August 4, 2022. https://www.washingtonpost.com/health/2022/08/04/monkeypox-public-health-emergency-united-states-becerra
- Minhaj FS, Ogale YP, Whitehill F, et al. ; Monkeypox Response Team 2022. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep 2022;71:764–9. https://doi.org/10.15585/mmwr.mm7123e1 PMID:35679181
- Thornhill JP, Barkati S, Walmsley S, et al. ; SHARE-net Clinical Group. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med 2022. Epub July 21, 2022. https://doi.org/10.1056/NEJMoa2207323 PMID:35866746
- US Census Bureau. Supplementary tables on race and Hispanic origin: 2020 census redistricting data. Washington, DC: US Department of Commerce, US Census Bureau; 2021. https://www2.census.gov/programs-surveys/decennial/2020/data/redistricting-supplementary-tables/redistricting-supplementary-table-04.pdf
- McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis 2014;58:260–7. https://doi.org/10.1093/cid/cit703 PMID:24158414
- Tarín-Vicente EJ, Agud-Dios M, Alemany A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain : a prospective cohort study. Rochester, NY: SSRN 2022. [Preprint posted July 18, 2022]. https://papers.ssrn.com/abstract=4162718
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ 2022;378:e072410. https://doi.org/10.1136/bmj-2022-072410 PMID:35902115
- O’Shea J, Filardo TD, Morris SB, Weiser J, Petersen B, Brooks JT. Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb Mortal Wkly Rep 2022;71. https://www.cdc.gov/mmwr/volumes/71/wr/mm7132e4.htm?s_cid=mm7132e4_w
 FIGURE. Monkeypox cases, by report date* — United States, May 17–July 22, 2022
FIGURE. Monkeypox cases, by report date* — United States, May 17–July 22, 2022
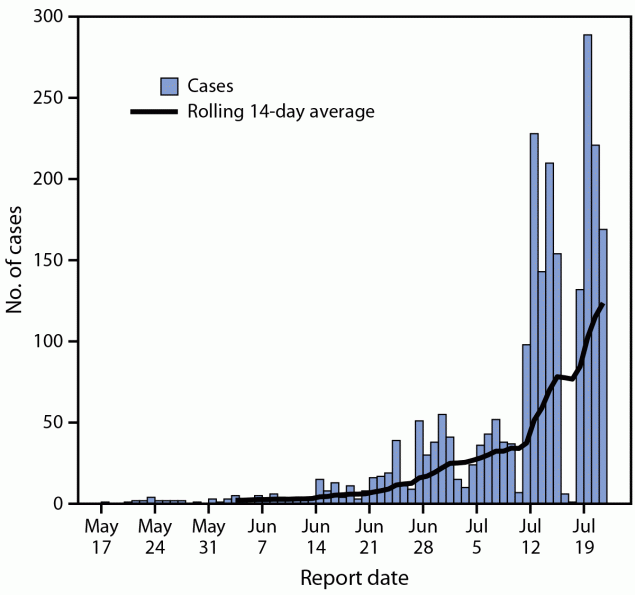
* Includes either the positive laboratory test report date, CDC call center reporting date, or date of case data entry into CDC’s emergency response common operating platform.
* Percentages calculated using nonmissing data.
* Symptoms experienced up until the time of interview.
† Symptoms reported by persons with monkeypox as their first symptoms during their illness or the body location where rash first appeared.
§ Percentages calculated using nonmissing data.
¶ Rash includes at least one lesion affecting the skin or mucous membranes.
Suggested citation for this article: Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and Clinical Characteristics of Monkeypox Cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1018-1022. DOI: http://dx.doi.org/10.15585/mmwr.mm7132e3.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.