Notes from the Field: Mucormycosis Cases During the COVID-19 Pandemic — Honduras, May–September 2021
Weekly / December 17, 2021 / 70(50);1747–1749
Homer Mejía-Santos1,*; Sandra Montoya2,*; Rafael Chacón-Fuentes3; Emily Zielinski-Gutierrez3; Beatriz Lopez3; Mariangeli F. Ning3; Nasim Farach3; Fany García-Coto4; David S. Rodríguez-Araujo4; Karla Rosales-Pavón1; Gustavo Urbina1; Ana Carolina Rivera1; Rodolfo Peña5; Amy Tovar5; Mitzi Castro Paz6; Roque Lopez6; Fabian Pardo-Cruz7; Carol Mendez7; Angel Flores7; Mirna Varela7; Tom Chiller8; Brendan R. Jackson8; Alexander Jordan8; Meghan Lyman8; Mitsuru Toda8; Diego H. Caceres8,9,†; Jeremy A. W. Gold8,† (View author affiliations)
View suggested citationAltmetric:
On July 15, 2021, the Secretary of Health of Honduras (SHH) was notified of an unexpected number of mucormycosis cases among COVID-19 patients. SHH partnered with the Honduras Field Epidemiology Training Program, the Executive Secretariat of the Council of Ministers of Health of Central America and the Dominican Republic (SE-COMISCA), Pan American Health Organization (PAHO), and CDC to investigate mucormycosis cases at four geographically distinct hospitals in Honduras.
Mucormycosis is a severe, often fatal disease caused by infection with angioinvasive molds belonging to the order Mucorales. Risk factors for mucormycosis include certain underlying medical conditions (e.g., hematologic malignancy, stem cell or solid organ transplantation, or uncontrolled diabetes) and the use of certain immunosuppressive medications (1). COVID-19 might increase mucormycosis risk because of COVID-19–induced immune dysregulation or associated medical treatments, such as systemic corticosteroids and other immunomodulatory drugs (e.g., tocilizumab), which impair the immune response against mold infections (2). In India, an apparent increase in mucormycosis cases (which was referred to by the misnomer “black fungus”) was attributed to COVID-19 (3).
For this investigation, a mucormycosis case was defined as laboratory identification of Mucorales by direct microscopy, culture, or histopathology in a patient with a clinical diagnosis of mucormycosis.§ Cases were considered COVID-19–associated if the patient received a positive test result for SARS-CoV-2 (the virus that causes COVID-19) or a COVID-19 diagnosis¶ during the period 60 days before to 14 days after mucormycosis diagnosis. Investigators traveled to the four hospitals (three public, and one private) during August 30–September 10, 2021, to ascertain mucormycosis cases and abstract medical record data using a standardized Epi Info (version 7.2.3.1; CDC) case report form. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.**
Seventeen persons received a diagnosis of mucormycosis during May 5–September 6, 2021; these included 11 persons with COVID-19–associated cases (Figure). Mucormycosis was confirmed by direct microscopy (16 cases), fungal culture (13 cases), or histopathology (three cases). The demographic features, underlying conditions, and mucormycosis clinical signs and symptoms were similar between patients with and without COVID-19. Most patients were male (nine); the median age was 54 years (IQR = 32–68 years). Diabetes was the most common underlying condition (12 patients), and two patients had hematologic malignancies; no other underlying immunosuppressive medical conditions were noted. During hospitalization, none of the patients with diabetes experienced diabetic ketoacidosis. The most frequent mucormycosis clinical signs and symptoms were rhino-orbital (12 patients) and cutaneous (four patients). The median interval between hospital admission and first positive test result for mucormycosis was 7 days (range = -8 to 21 days). Among the 11 patients with COVID-19–associated mucormycosis cases, nine were unvaccinated against COVID-19; the median interval between COVID-19 diagnosis and the first positive test result for mucormycosis was 11 days (range = -12 to 58 days). Seven COVID-19 patients received supplemental oxygen therapy, nine received corticosteroids, and four received tocilizumab.
Ten of the 17 patients died during hospitalization, including eight of the 11 with COVID-19–associated mucormycosis; three patients remained hospitalized at the time of medical chart abstraction. Two of the seven surviving patients experienced major sequelae from mucormycosis, including facial disfiguration and limb loss.
The findings in this report are subject to at least two limitations. First, the actual extent of COVID-19–associated mucormycosis in Honduras is likely underrepresented because case investigations involved only four hospitals in the country. Second, because mucormycosis reporting is not required in Honduras, it is difficult to determine whether the cases described in this report represent an increase over the country’s baseline mucormycosis incidence, which is unknown. The primary laboratory for mycology in Honduras (population approximately 9,900,000)†† usually identifies approximately two mucormycosis cases annually (S. Montoya, Hospital Escuela, personal communication, October 2021). By comparison, the 17 mucormycosis cases described in this report occurred during approximately 4 months (May 5–September 6, 2021), coinciding with Honduras’s mid-year COVID-19 surge.§§,¶¶ This apparent increase in laboratory-identified mucormycosis cases might be related to the COVID-19 surge because of COVID-19–induced immune dysregulation or associated medical treatments (2). Alternatively, it might reflect the use of an active case-finding strategy during the investigation period. Increased case detection might also be related to higher clinician awareness and testing for mucormycosis, prompted by educational webinars held by SHH, SE-COMISCA, PAHO, and CDC after the initial detection of COVID-19–associated mucormycosis cases in Honduras.
Given the severe outcomes associated with mucormycosis, clinicians should remain vigilant for this disease during the COVID-19 pandemic, including in immunocompetent patients. Early mucormycosis diagnosis is possible, even in resource-limited settings (4). Mucormycosis treatment guidelines recommend prompt antifungal therapy*** and surgical intervention to reduce mortality (4). Prevention of COVID-19 through vaccination, maintenance of glycemic control in patients with diabetes, and judicious use of steroids†††,§§§ for COVID-19 treatment might help decrease the risk for mucormycosis associated with COVID-19 (2). Because of these reported cases, SHH and partners are conducting clinician outreach and education to improve prevention, diagnosis, and treatment of mucormycosis.
Acknowledgments
Diana Cassisi, Hospital Nacional Mario Catarino Rivas, San Pedro Sula, Honduras; Gustavo Avelar, Hospital General del Sur, Choluteca, Honduras; Saúl Soto, Hospital Escuela, Tegucigalpa, Honduras; Diana Varela, Hospital Escuela, Tegucigalpa, Honduras; Elsa Palou, Faculty of Medical Sciences, National Autonomous University of Honduras, Tegucigalpa, Honduras; CDC COVID-19 International Task Force.
Corresponding author: Jeremy A.W. Gold, jgold@cdc.gov.
1Health Surveillance Unit, Secretary of Health of Honduras, Tegucigalpa, Honduras; 2Department of Mycology, Hospital Escuela, Tegucigalpa, Honduras; 3Central America Regional Office, CDC; 4Council of Ministers of Health of Central America and the Dominican Republic, Tegucigalpa, Honduras; 5Honduras Country Office, Pan American Health Organization, Tegucigalpa, Honduras; 6National Public Health Laboratory, Secretary of Health of Honduras, Tegucigalpa, Honduras; 7Honduras Field Epidemiology Training Program, Tegucigalpa, Honduras; 8Mycotic Diseases Branch, CDC; 9Department of Medical Microbiology, Radboud University Medical Center and Center of Expertise in Mycology Radboudumc/Canisius Wilhelmina Ziekenhuis, Nijmegen, The Netherlands.
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
* These authors contributed equally to this report.
† These authors contributed equally to this report.
§ Signs and symptoms of mucormycosis vary by the affected body site. Rhino-orbital-cerebral mucormycosis signs and symptoms frequently include unilateral facial swelling, headache, sinus congestion, and necrotic lesions of the nasal bridge or palate. Cutaneous mucormycosis signs and symptoms frequently include blisters or ulcers that become necrotic, and pain, erythema, or swelling around a wound. Pulmonary mucormycosis signs and symptoms frequently include cough, chest pain, and shortness of breath. https://www.cdc.gov/fungal/diseases/mucormycosis/symptoms.html
¶ A COVID-19 case was defined as receipt of a positive SARS-CoV-2 reverse transcription–polymerase (RT-PCR) chain reaction or antigen test result, or a clinical diagnosis of COVID-19 in a patient who received a positive serologic test result (RT-PCR or antigen testing was not available in some areas).
** 45 C.F.R. part 46.102(l)(2), 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq.
†† https://population.un.org/wpp/Download/Standard/Population/
§§ https://covid19.who.int/region/amro/country/hn (Accessed December 9, 2021).
¶¶ http://covid19honduras.org/(Accessed December 9, 2021).
*** Antifungal drugs that are effective against mucormycosis include amphotericin B, posaconazole, and isavuconazole. Other antifungal drugs, including fluconazole, voriconazole, and echinocandins, are not effective for treating mucormycosis.
††† https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1
References
- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis 2012;54(Suppl 1):S16–22. https://doi.org/10.1093/cid/cir865 PMID:22247441
- Narayanan S, Chua JV, Baddley JW. COVID-19 associated Mucormycosis (CAM): risk factors and mechanisms of disease. Clin Infect Dis 2021. Epub August 22, 2021. https://doi.org/10.1093/cid/ciab726 PMID:34420052
- Patel A, Agarwal R, Rudramurthy SM, et al. MucoCovi Network3. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis 2021;27:2349–59. https://doi.org/10.3201/eid2709.210934 PMID:34087089
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019;19:e405–21. https://doi.org/10.1016/S1473-3099(19)30312-3 PMID:31699664
 FIGURE. Time line of diagnosis, treatment, and outcomes for patients hospitalized with mucormycosis (N = 17) — Honduras, May–September 2021*
FIGURE. Time line of diagnosis, treatment, and outcomes for patients hospitalized with mucormycosis (N = 17) — Honduras, May–September 2021*
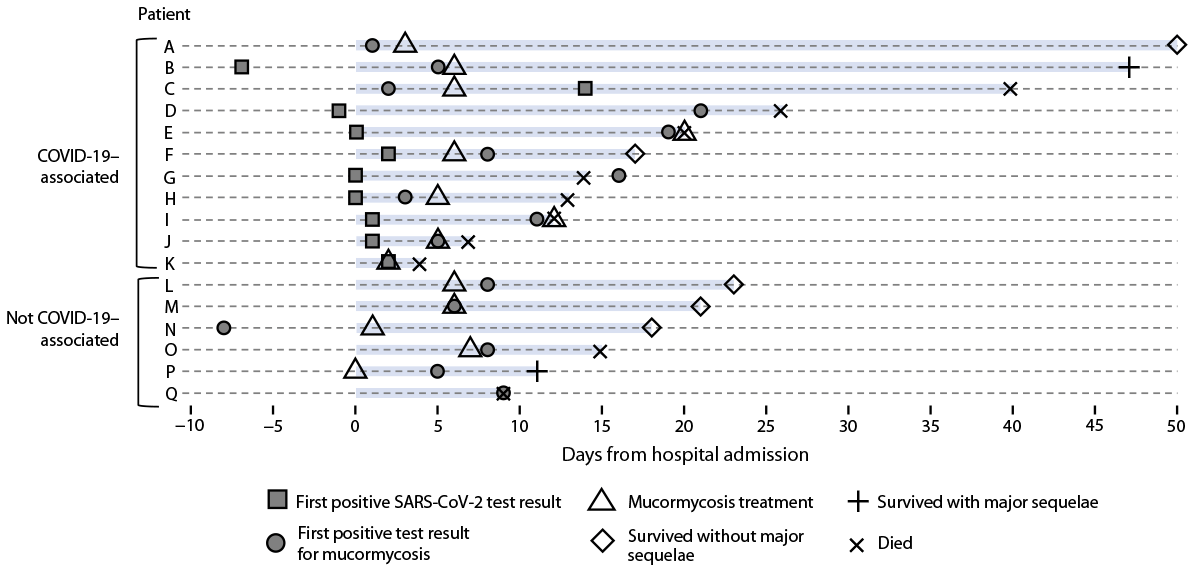
* Additional patient information: patient A’s COVID-19 diagnosis date (not included) occurred 58 days before the date of the first positive mucormycosis test result; patients B, F, and M remained hospitalized on the date of data abstraction; and patient K’s date of first positive mucormycosis test result was unavailable.
Suggested citation for this article: Mejía-Santos H, Montoya S, Chacón-Fuentes R, et al. Notes from the Field: Mucormycosis Cases During the COVID–19 Pandemic — Honduras, May–September 2021. MMWR Morb Mortal Wkly Rep 2021;70:1747–1749. DOI: http://dx.doi.org/10.15585/mmwr.mm7050a2.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.