What to know
- CDC works with state and local public health departments, clinicians, travel industry partners, and others to identify and test people who may be infected with Middle East respiratory syndrome coronavirus (MERS-CoV).
Testing for MERS-CoV
CDC recommends that clinicians and public health departments consider a full differential diagnosis, and order pathogen-specific testing based on the most likely etiology for the patient's clinical presentation. In most scenarios in the United States, this likely indicates testing for other more common pathogens before testing for MERS-CoV; the pre-test probability is likely higher for most other respiratory viruses and/or common bacterial pathogens.
If there is strong suspicion that a person under investigation (PUI) is infected with MERS-CoV, simultaneous testing for MERS-CoV along with other possible pathogens can be considered following discussion with state and local health departments.
Healthcare providers should consider testing for:
- Common viral respiratory pathogens, including SARS-CoV-2, influenza A, influenza B, respiratory syncytial virus (RSV), human metapneumovirus, human parainfluenza viruses, seasonal human coronaviruses, adenovirus, enterovirus/rhinovirus, and other respiratory viruses
- Streptococcus pneumoniae, Chlamydia pneumophila, Legionella pneumophila, Mycoplasma pneumoniae, and other bacterial pathogens that cause severe lower respiratory infections
How to request laboratory testing
Multiple public health laboratories offer diagnostic MERS-CoV PCR testing, which is a type of nucleic acid amplification test (NAAT). Contact your local/state health department to notify them of the PUI and to request MERS-CoV testing. If your state health department is unable to test but feels testing is warranted, they should contact CDC's Emergency Operations Center, for CDC consultation and to request CDC MERS-CoV testing. Pre-approval is required before CDC can perform MERS-CoV testing.
Types of tests
Molecular Tests
Molecular tests diagnose active infection (presence of MERS-CoV). NAATs such as real-time reverse-transcription polymerase chain reaction (rRT-PCR) assays are molecular tests that detect viral genetic material in clinical specimens.
CDC developed an rRT-PCR assay that is the primary test for MERS diagnostic purposes in the United States. FDA granted Emergency Use Authorization for this test.
Specimen Types for Molecular Tests
CDC recommends collecting and testing multiple specimens for the rRT-PCR assay, including lower (bronchoalveolar lavage, sputum and tracheal aspirates) and upper (nasopharyngeal and oropharyngeal swabs) respiratory specimens. For patients with possible MERS-CoV pneumonia, lower respiratory specimens are the preferred specimen type, because they have higher test sensitivity.
Specimen collection overview
Before collecting and handling specimens for MERS-CoV testing, determine whether the person meets the current definition for a PUI for MERS-CoV infection.
Specimen Type and Priority
Proper specimen collection is the most important step in the laboratory diagnosis of infectious diseases. CDC recommends collecting one or multiple specimens from each site after symptom onset, if possible. Specimen types include:
- Lower respiratory (bronchoalveolar lavage, sputum and tracheal aspirates)
- Upper respiratory (nasopharyngeal and oropharyngeal swabs)
Whenever possible, lower respiratory tract specimens are preferred. However, in patients who are unable to produce sputum or who are not severely ill, testing nasopharyngeal and oropharyngeal specimens is possible.
Respiratory specimens should be collected as soon as possible, ideally within 7 days of symptom onset. However, if more than a week has passed since symptom onset and the patient is still symptomatic, respiratory specimens should still be collected, especially lower respiratory tract specimens.
Specimens can be stored at 2-8°C for up to 72 hours after collection. If extraction cannot be completed within 72 hours, specimens should be stored at -70°C or lower as soon as possible after collection. Label specimen containers with two primary patient identifiers, in accordance with Clinical Laboratory Improvement Amendments (CLIA) regulations for diagnostic testing: specimen type and the date the specimen was collected. If specimens are being sent to the local or state public health laboratory, contact their laboratory for instruction on collection and shipment. If submitting specimens to CDC for MERS-CoV testing, detailed instructions are provided in the CDC-10488 test order.
Respiratory Specimen Collection
1. Lower respiratory tract
Bronchoalveolar lavage, tracheal aspirate – Collect 2-3 mL into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container.
Sputum – Have the patient rinse the mouth with water and then expectorate deep cough sputum directly into a sterile, leak-proof, screw-cap sputum collection cup or sterile dry container. A minimum of 200 µL is required for testing.
2. Upper respiratory tract -- nasopharyngeal (NP) and oropharyngeal (OP) swabs
Nasopharyngeal swab - Tilt patient's head back 70 degrees. Gently insert a swab into the nostril parallel to the palate and advance to enter the nasopharyngeal space. Gently rub and roll the swab. Leave the swab in place for a few seconds to absorb secretions. Remove the swab and immediately place tip first into sterile tube containing 2-3 mL of transport media.
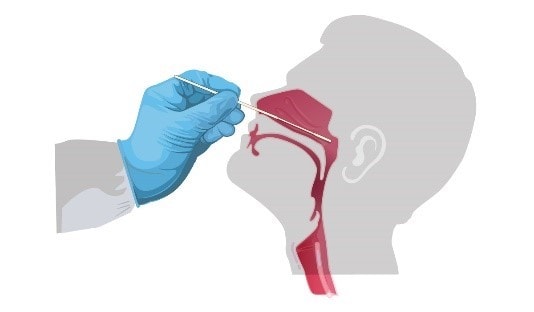
Oropharyngeal swab (e.g., throat swab) – Insert swab into the posterior pharynx and tonsillar areas, avoiding the tongue. Immediately place swab tip first into sterile tubes containing 2-3 mL of transport media.
Use only synthetic fiber swabs with thin plastic or wire shafts. Do not use calcium alginate swabs or swabs with wooden shafts, as they may contain substances that inactivate some viruses and inhibit PCR testing. Place swabs immediately into sterile tubes containing 2-3 mL of transport media. As there are various types of transport media, contact the testing laboratory to determine which transport media types can be accepted prior to specimen collection.
After collection, NP and OP specimens can be combined, placing both swabs in the same vial.
Sending specimens
Package, ship, and transport specimens from suspected MERS PUIs according to the current edition of the International Air Transport Association (IATA) Dangerous Goods Regulations. Shipments from outside the United States may require an importation permit that can be obtained from CDC.
Specimens must be shipped on dry ice. When shipping frozen specimens from long distances or from international locations, use sufficient dry ice to ensure specimens remain frozen during the entire transport process.
All specimens must be prepared in a manner that prevents the risk of breakage and spillage during transport. Seal specimen containers with Parafilm® (primary container) and place in zippered plastic bags (secondary container). Ensure sufficient absorbent material is placed in the secondary container to absorb the entire contents if a leakage occurs. Whenever possible, separate the primary containers (containing specimen) to reduce any potential risk of breakage. When shipping large numbers of specimens, organize them in a sequential manner in boxes with separate compartments for each specimen.
Additional recommendations:
- Do not place any dry ice in the primary container or secondary container, foam envelopes, zippered plastic bags, cryovial boxes, or hermetically sealed containers.
- Do not place primary containers sideways or upside down in zippered plastic bags.
- Do not place any paperwork in the secondary containers or zippered plastic bags, so as not to damage the paperwork.
- Do not use biohazard/autoclave bags to prepack your materials because of the inadequate seal of these bags.
For additional information or consultation, contact the CDC Emergency Operations Center. If shipping to a local or state public health laboratory, contact the laboratory directly for instructions. CDC's shipping address and instructions are available on the CDC Test Directory for MERS-CoV Molecular Detection. Ship specimens for overnight delivery. If weekend delivery is planned, special arrangements must be made with the shipping company and with CDC.
Interpreting results
A MERS diagnosis requires a positive test result from a molecular test, which indicates that MERS-CoV genetic material was detected in a patient's clinical specimen. Until confirmatory testing is performed, a positive result is considered a presumptive positive. Refer to the specific test's instructions for use for details on how to interpret test results.
Interpretation of a negative rRT-PCR test result depends on multiple factors, including specimen type and time since illness onset. Collecting and testing multiple specimens may increase confidence that the person under investigation does not have an active infection.
Laboratory guidelines
Biosafety Guidelines for Handling and Processing Specimens Associated with MERS-CoV
Biosafety precautions should be taken in collecting and handling specimens that may contain MERS-CoV. Timely communication between clinical and laboratory staff is essential to minimize the risk incurred in handling specimens from patients with possible MERS-CoV infection. Label such specimens accordingly and alert the receiving laboratory to ensure proper specimen handling. General and specific biosafety guidelines for handling MERS-CoV specimens are provided below.
For additional detailed instructions, please refer to the following:
- Biosafety in Microbiological and Biomedical Laboratories (BMBL) - 6th Edition
- Laboratory Biosafety Manual - 3rd Edition
- Prevention and Control for Hospitalized MERS Patients
Any procedure with the potential to generate fine-particulate aerosols (e.g., vortexing or sonication of specimens in an open tube) should be performed in a Class II Biological Safety Cabinet (BSC). Appropriate physical containment devices (e.g., centrifuge safety buckets, sealed rotors) should be used for centrifugation. Ideally, rotors and buckets should be loaded in a BSC. Perform any procedures outside a BSC in a manner that minimizes the risk of exposure to a pathogen as a result of an inadvertent specimen release.
After specimens are processed, decontaminate work surfaces and equipment with appropriate disinfectants. Use any EPA-registered hospital disinfectant. Follow manufacturer's recommendations for use, including dilution (i.e., concentration), contact time, and care in handling.
Autoclave all disposable waste.
Specific Guidelines for Handling MERS-CoV in Laboratory Settings
Clinical and microbiology laboratories should follow standard laboratory practices, including Standard Precautions, when handling potential MERS-CoV specimens. For additional information, see Biosafety in Microbiological and Biomedical Laboratories (BMBL) - 6th Edition.
The following activities must be performed in a BSL-3 facility using BSL-3 work practices:
- Propagating MERS-CoV in cell culture
- Characterizing viral agents recovered in cultures of MERS-CoV specimens
The following activities must be performed in Animal BSL-3 facilities using Animal BSL-3 work practices:
- Inoculating animals for potential recovery of virus from MERS-CoV specimens
- Inoculating animals for characterization of MERS-CoV
- National Center for Immunization and Respiratory Diseases (NCIRD) | NCIRD | CDC
