At a glance
Introduction
Accurate and timely medical diagnoses support public health and patient care. In a 2015 report, "Improving Diagnosis in Healthcare," the National Academies of Medicine identified diagnostic error as a major public health challenge.1 Annually, approximately 800,000 Americans die or become disabled due to diagnostic errors associated with serious conditions that include cancer, cardiovascular, and infectious diseases.12 These diagnostic errors contribute to about one-third of all medical errors.
Keep in mind
The Diagnostic Excellence Initiative within the CDC Division of Laboratory Systems was established in 2022 to advance the inclusion of laboratory expertise and capabilities in healthcare delivery to reduce diagnostic errors and improve accurate, timely, and actionable diagnoses.3 Many of our projects and activities are described below.
The Total Testing Process
The Total Testing Process provides a framework for engaging with partners to advance diagnostic excellence and support the Diagnostic Excellence Initiative projects and activities described below.4
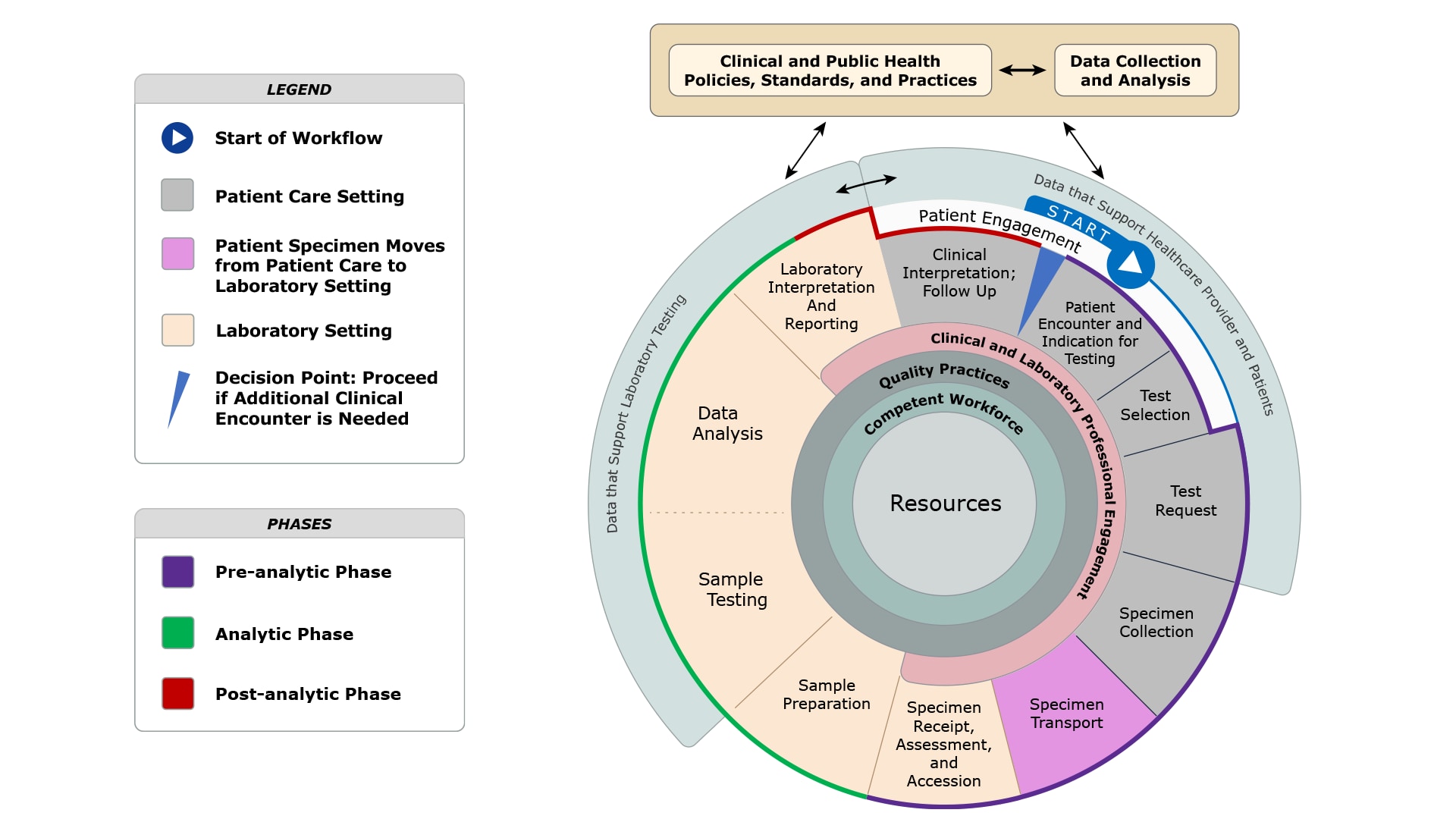
- Pre-analytic Phase: Steps that occur prior to testing of the patient sample that begins with an indication for testing and concludes with specimen receipt, assessment, and accession.
- Analytic Phase: Steps that occur during testing of the patient sample that includes sample preparation, sample testing, and subsequent data analysis, laboratory interpretation and reporting.
- Post-analytic Phase: Steps that occur after testing of the patient sample that includes clinical interpretation and follow-up.
Projects and activities
Learn more about our projects and activities that support diagnostic excellence:
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Data that Support Healthcare Providers and Patients
- Clinical and Laboratory Professional Engagement
- Quality Practices
- Competent Workforce
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Outcomes Sought
Collaborators
- CDC Division of Heart Disease and Stroke Prevention
- National Association for Community Health Centers (NACHC)
- Million Hearts Initiative
- Zufall Health
- Health Efficient
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
Outcomes sought
Collaborators
Project Period
- Specimen Collection
- Quality Practices
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Clinical and Public Health Policies, Standards, and Practices
- Data Collection and Analysis
- Resources
Outcomes Sought
- Increased adoption of the blood culture contamination (BCC) measure as a quality monitor among laboratory accreditation organizations.
- Improved data collection of laboratory quality indicators for BCC.
- Appropriate collection of blood cultures among hospitals and healthcare systems.
- Improved communication, follow-up, and training among clinical laboratories and clinical care teams in collaboration with antibiotic and diagnostic stewardship teams.
- CDC Division of Healthcare Quality Promotion
- National Healthcare Safety Network
- December 2022: Initial Measure Endorsement:
- FY 2025: Measure maintenance/renewal application due Spring 2026 cycle
- National Healthcare Safety Network
- Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals
- Blood Culture Contamination: An Overview for Infection Control and Antibiotic Stewardship Programs Working with the Clinical Laboratory
- Adult Blood Culture Contamination Rate; A national measure and standard for clinical laboratories and antibiotic stewardship programs
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Resources
- CDC Office of Genomics and Precision Public Health
- CDC Office of Advanced Molecular Detection
- PRPD Diagnostics
- Laboratory Interpretation and Reporting
- Clinical Interpretation and Follow-up
- Data that Support Healthcare Providers and Patients
- Clinical and Laboratory Professional Engagement
- Quality Practices
- Competent Workforce
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Presence of eGFRs in the patient record when blood creatinine levels are recorded.
- Use of the CKD-EPI 2021 equation to calculate eGFRs.
- Low eGFRs are followed up with a repeat test to identify CKD.
- Indication of CKD for patients having chronic low eGFRs.
- Use of urine albumin to creatine ratio to detect proteinuria for low eGFRs.
- CDC Division of Diabetes Translation
- National Kidney Foundation
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Test Selection
- Clinical and Public Health Policies, Practices, and Standards
- Data Collection and Analysis
- Resources
- Clinical and Public Health Policies, Practices, and Standards
Outcomes Sought
Collaborators
Project Period
Resources
- Clinical Laboratory Improvement Advisory Committee (CLIAC) Recommendations Table (Oct 28-29, 2018; Nov 7-8, 2018; Nov 1-2, 2017; Nov 2-3, 2016)
- Patient Engagement
- Resources
Outcomes Sought
Project Period
Resources
- CDC Chronic Kidney Disease
- NIH Chronic Kidney Disease
- National Kidney Foundation
- Improving Your Laboratory Testing Process | Agency for Healthcare Research and Quality
- Implementing a safer and more reliable system to monitor test results at a teaching university-affiliated facility in a family medicine group: a quality improvement process report | BMJ Open Quality
- Improving Diagnosis in Healthcare
- Burden of Serious Harms from Diagnostic Error in the USA
- Bringing the Clinical Laboratory into the Strategy to Advance Diagnostic Excellence
- The Clinical Laboratory Is an Integral Component to Health Care Delivery: An Expanded Representation of the Total Testing Process
- Prevalence, Awareness, and Treatment of Elevated LDL Cholesterol in US Adults
- Advancing Diagnostic Stewardship for Healthcare-Associated Infectious, Antibiotic Resistance, and Sepsis
- Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem
- National Kidney Foundation Laboratory Engagement Working Group Recommendations for Implementing the CKD-EPI 2021 Race-Free Equations for Estimated Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories
- Reported Awareness and Adoption of 2021 Estimated Glomerular Filtration Rate Equations Among US Clinical Laboratories
