What to know
The information on this page should be considered the latest for the current influenza season for clinical practice regarding the use of influenza antiviral medications.
Priority Groups for Antiviral Treatment of Influenza
Antiviral treatment is recommended as soon as possible for any patient with suspected or confirmed influenza who:
- is hospitalized;
- has severe, complicated, or progressive illness; or
- is at higher risk for influenza complications.
Decisions about starting antiviral treatment for patients with suspected influenza should not wait for laboratory confirmation of influenza virus infection. Empiric antiviral treatment should be started as soon as possible in the above priority groups.
Clinicians can consider early empiric antiviral treatment of non-higher-risk outpatients with suspected influenza based upon clinical judgment if treatment can be initiated within two days of illness onset.
Antiviral Drug Options
- For hospitalized patients with suspected or confirmed influenza, initiation of antiviral treatment with oral or enterically administered oseltamivir is recommended as soon as possible.
- For outpatients with complications or progressive disease >2 days after symptom onset and suspected or confirmed influenza (e.g., pneumonia, or exacerbation of underlying chronic medical conditions), initiation of antiviral treatment with oral oseltamivir is recommended as soon as possible.
- For outpatients with suspected or confirmed uncomplicated influenza within two days of symptom onset, oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir may be used for treatment, depending upon approved age groups and contraindications. In one randomized controlled trial, baloxavir had greater efficacy than oseltamivir in adolescents and adults with influenza B virus infection (Ison, 2020).
Clinicians may also wish to consult:
Co-circulation of Influenza Viruses and SARS-CoV-2
During periods of community co-circulation of influenza viruses and SARS-CoV-2, empiric antiviral treatment of influenza is recommended as soon as possible for the following priority groups: a) hospitalized patients with respiratory illness; b) outpatients with severe, complicated, or progressive respiratory illness; and c) outpatients at higher risk for influenza complications who present with any acute respiratory illness symptoms (with or without fever).
- Influenza and COVID-19 have overlapping signs and symptoms. Testing can help distinguish between influenza virus infection and SARS-CoV-2 infection. However, clinicians should not wait for the results of influenza testing (Nucleic Acid Detection Based Tests), SARS-CoV-2 testing, or multiplex molecular assays that detect influenza A and B viruses and SARS-CoV-2 (Multiplex Assays Authorized for Simultaneous Detection of Influenza Viruses and SARS-CoV-2 by FDA) to initiate empiric antiviral treatment for influenza in the above priority groups.
- Co-infection with influenza A or B viruses and SARS-CoV-2 can occur and should be considered, particularly in hospitalized patients with severe respiratory disease.
- Clinicians should be aware that a positive SARS-CoV-2 test result does not preclude influenza virus infection. For hospitalized patients with suspected influenza who are started on empiric antiviral treatment with oseltamivir, use of influenza molecular assays (Nucleic Acid Detection Based Tests) or multiplex assays that detect both influenza viruses and SARS-CoV-2 (Multiplex Assays Authorized for Simultaneous Detection of Influenza Viruses and SARS-CoV-2 by FDA) can inform clinical management.
- Clinicians should be aware that a positive influenza test result does not preclude SARS-CoV-2 infection. For hospitalized patients with a positive influenza test result, antiviral treatment of influenza with oseltamivir should be started as soon as possible, and clinicians should also follow guidelines for diagnosis and treatment of community-acquired pneumonia (community acquired pneumonia treatment guidance for adults: Metlay, 2019) and other respiratory infections, including SARS-CoV-2 infection (IDSA Guidelines on the Treatment and Management of Patients with COVID-19) if clinically indicated, while awaiting SARS-CoV-2 testing results. Oseltamivir does not have in-vitro activity against SARS-CoV-2 (Choy, 2020).
- Clinicians can utilize telemedicine in place of office visits for patients with acute respiratory illness. It may be useful for providers to implement phone triage lines to enable high-risk patients to discuss symptoms over the phone. Algorithm to Assist in Medical Office Telephone Evaluation of Patients with Possible Influenza for Health Care Settings.
- Patients at higher risk for influenza complications should be advised to call their provider as soon as possible if they have acute respiratory illness symptoms (with or without fever) for consideration of infection with influenza A or B viruses (and early antiviral treatment), SARS-CoV-2, and other respiratory pathogens.
- Clinicians can consider starting early (within two days of illness onset) empiric antiviral treatment of non-higher-risk outpatients with suspected influenza, based upon clinical judgment, including without an office visit. SARS-CoV-2 and other etiologies of acute respiratory illness should also be considered.
- Infectious Diseases Society of America (IDSA) Guidelines on the Treatment and Management of Patients with COVID-19 are available.
- Clinical algorithms for the testing and treatment of influenza when SARS-CoV-2 and influenza viruses are circulating are available.
Overview of Influenza Antiviral Medications
Antiviral medications with activity against influenza viruses are an important adjunct to influenza vaccine in the control of influenza.
- Influenza antiviral prescription drugs can be used to treat influenza, and some can be used to prevent influenza.
- Six licensed prescription influenza antiviral drugs are approved in the United States.
- Four influenza antiviral medications approved by the U.S. Food and Drug Administration (FDA) are recommended for use in the United States.
- Three drugs are chemically related antiviral medications known as neuraminidase inhibitors that block the viral neuraminidase enzyme and have activity against both influenza A and B viruses: oral oseltamivir phosphate (available as a generic version or under the trade name Tamiflu®), inhaled zanamivir (trade name Relenza®), and intravenous peramivir (trade name Rapivab®).
- The fourth drug is oral baloxavir marboxil (trade name Xofluza®), which is active against both influenza A and B viruses but has a different mechanism of action than neuraminidase inhibitors. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication.
- More information regarding the four recommended antiviral medications is available: Table 1.
- The two other FDA-approved influenza antiviral medications (amantadine and rimantadine) are not recommended for antiviral treatment or chemoprophylaxis because of high levels of resistance among circulating influenza A viruses.
- Clinical trials and observational data show that early antiviral treatment can shorten the duration of fever and illness symptoms, and may reduce the risk of some complications from influenza (e.g., otitis media in young children, pneumonia, and respiratory failure).
- In hospitalized adults with influenza, early treatment with oseltamivir has been reported to reduce in-hospital death and the duration of hospitalization in some observational studies.
- In hospitalized children, early antiviral treatment with oseltamivir has been reported to shorten the duration of hospitalization in some observational studies.
- Clinical benefit is greatest when antiviral treatment is administered early, especially within 48 hours of influenza illness onset.
Antiviral Medications Recommended for Treatment and Chemoprophylaxis of Influenza
Abbreviations: N/A = not applicable, COPD = chronic obstructive pulmonary disease.
- Oral oseltamivir phosphate is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 14 days and older, and for chemoprophylaxis in people 1 year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by the CDC and the American Academy of Pediatrics. If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical due to limited data in this age group. Dose adjustment is recommended for patients with reduced creatinine clearance. More information is available in the "Dosing in Adult Patients with Renal Impairment" section and Table 3.
- Self-injury or delirium; mainly reported among Japanese pediatric patients.
- Inhaled zanamivir is contraindicated in patients with underlying airways disease such as asthma or chronic obstructive pulmonary disease, and those with a history of allergy to lactose or milk protein.
- Intravenous peramivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 6 months and older. Peramivir efficacy is based on clinical trials versus placebo in which the predominant influenza virus type was influenza A; in one trial, a very limited number of subjects infected with influenza B virus were enrolled. Dose adjustment is recommended for patients with reduced creatinine clearance. More information is available in the "
- There are no data available for use of peramivir for chemoprophylaxis of influenza.
- Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people aged ≥5 years who are otherwise healthy or who are high risk of developing influenza-related complications. Baloxavir efficacy for initial FDA approval in October 2018 was based on clinical trials in previously healthy outpatients 12 to 64 years old (Hayden, 2018). Single-dose baloxavir treatment was superior to placebo and had similar clinical efficacy in time to alleviation of symptoms to a 5-day treatment course of oseltamivir.
In October 2018, FDA approved baloxavir for the treatment of acute uncomplicated influenza in patients 12 years and older who had been symptomatic no more than 48 hours.
In October 2019, FDA approved an indication for baloxavir treatment of acute uncomplicated influenza within 48 hours of illness onset in people 12 years and older at high risk of developing influenza-related complications, based upon the findings of a clinical trial (Ison, 2020). In this clinical trial of early initiation of antiviral treatment for uncomplicated influenza in higher risk patients, baloxavir was superior to placebo and had similar overall efficacy to oseltamivir in the time to alleviation of symptoms. For patients with influenza B virus infection, baloxavir significantly reduced the median time to improvement of symptoms compared with oseltamivir by more than 24 hours.
In August 2022, FDA expanded approval of baloxavir for treatment of acute uncomplicated influenza within 48 hours of illness onset in children aged 5 years to <11 years who are otherwise healthy package insert XOFLUZA. This was based upon the secondary clinical outcomes of a randomized clinical trial of baloxavir versus oseltamivir for treatment of uncomplicated influenza in children aged 1 year to <12 years (Baker, 2021).
In November 2020, FDA expanded approval of baloxavir to include post exposure prophylaxis of influenza for persons aged ≥12 years within 48 hours of contact with an individual with influenza, based on the findings of a clinical trial among household contacts of index patient with influenza (Ikematsu, 2020). In this study, baloxavir post-exposure prophylaxis (PEP) of influenza in household members (19% were younger than 12 years; 73% received baloxavir within 24 hours of onset of symptoms in the index household case who received antiviral treatment) significantly reduced the risk of laboratory confirmed by 86% among those who received baloxavir PEP than among those who received placebo (1.9% [7 of 374] vs. 13.6% [51 of 375]; adjusted risk ratio, 0.14; 95% confidence interval [CI], 0.06 to 0.30; P<0.001).
In August 2022, FDA expanded approval of baloxavir for post-exposure prophylaxis of influenza in persons aged 5 years and older within 48 hours of contact with an individual with influenza package insert XOFLUZA.
There are no available data for baloxavir treatment for influenza during pregnancy, in immunocompromised people, or in those with severe influenza who are not hospitalized. A randomized clinical trial reported that combination neuraminidase inhibitor (primarily oseltamivir) and baloxavir for treatment of hospitalized influenza patients aged ≥12 years did not result in superior clinical benefit (time to clinical improvement) compared with neuraminidase inhibitor and placebo (Kumar, 2022).
Summary of Influenza Antiviral Treatment Recommendations
- Antiviral treatment is recommended as early as possible for any patient with confirmed or suspected influenza who:
- Oral oseltamivir is the preferred treatment during pregnancy (Rasmussen, 2009; Rasmussen, 2011). The same antiviral dosing is recommended during pregnancy as for those who are not pregnant. Multiple studies have reported safe use of neuraminidase inhibitors during pregnancy (Dunstan, 2014; Xie, 2013; Saito, 2013; Wollenhaupt, 2014; Beau, 2014; Svensson, 2011; Greer, 2010; Graner, 2017; Ehrenstien, 2018; Chambers, 2019; Bennekom, 2019; ACOG Committee, 2018). Recommendations for Obstetric Health Care Providers Related to Use of Antiviral Medications in the Treatment and Prevention of Influenza for additional information. Baloxavir is not recommended for the treatment of influenza during pregnancy or while breastfeeding, as there are no available efficacy or safety data for baloxavir in pregnancy (Chow, 2021), and no available data on the presence of baloxavir in human milk, the effects on the breastfed infant, or the effects on milk production.
- CDC does not recommend use of baloxavir for monotherapy of influenza in severely immunosuppressed persons. There are no available efficacy, safety, or resistance data for baloxavir monotherapy of influenza in severely immunosuppressed patients and emergence of resistance during treatment is a concern because of prolonged influenza viral replication in these patients.
- When indicated, antiviral treatment should be started as soon as possible after illness onset, ideally within 48 hours of symptom onset for the greatest clinical benefit. However, observational studies have reported that antiviral treatment of influenza can have clinical benefit in patients with severe, complicated or progressive illness, and in hospitalized patients when started after 48 hours of illness onset.
- Decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza.
- Clinical benefit is greatest when antiviral treatment is started as close to illness onset as possible.
- Antiviral treatment with oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir also can be considered for any previously healthy, symptomatic outpatient not at higher risk for influenza complications, who is diagnosed with confirmed or suspected influenza, on the basis of clinical judgment, if treatment can be initiated within two days of illness onset.
- The recommended treatment course for uncomplicated influenza is two doses per day of oral oseltamivir or inhaled zanamivir for five days, or one dose of intravenous peramivir or oral baloxavir for one day.
- While influenza vaccination is the best way to prevent influenza illness, a history of influenza vaccination does not rule out the possibility of influenza virus infection in an ill patient with clinical signs and symptoms compatible with influenza.
Figure: Guide for considering influenza testing and treatment when influenza viruses are circulating in the community (regardless of influenza vaccination history)1
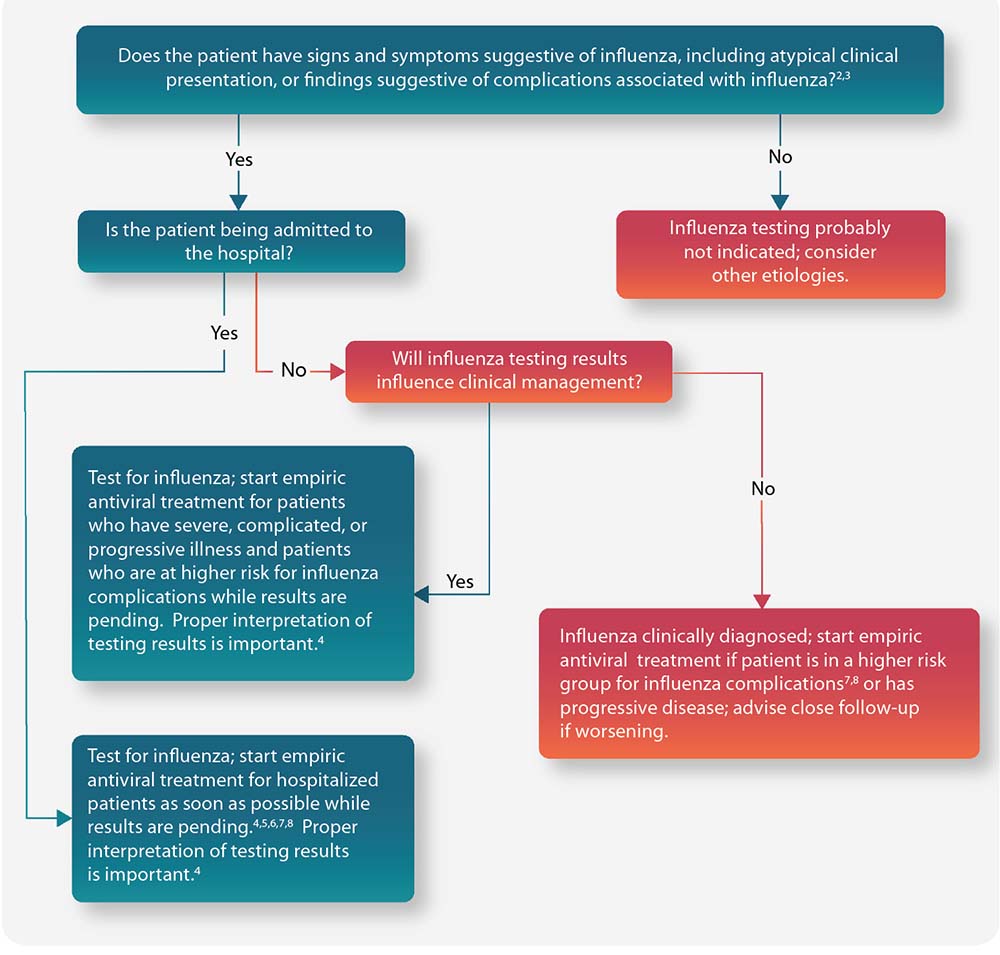
People at Higher Risk for Influenza Complications Recommended for Antiviral Treatment
Treatment Considerations for Patients Hospitalized with Suspected or Confirmed Influenza
The following recommendations do not necessarily represent FDA-approved uses of antiviral products but are based on published observational studies and expert opinion and are subject to change as the developmental status of investigational products and the epidemiologic and virologic features of influenza change over time.
- For hospitalized patients with suspected or confirmed influenza, initiation of antiviral treatment with oral or enterically administered oseltamivir is recommended as soon as possible. Antiviral treatment might be effective in reducing morbidity and mortality in hospitalized influenza patients, especially adults, even if treatment is started more than 48 hours after onset of illness.
- Inhaled zanamivir, oral baloxavir, and intravenous peramivir are not recommended routinely for hospitalized patients with suspected or confirmed influenza because of insufficient data on use of these antivirals showing clinical benefit in hospitalized influenza patients. There are also insufficient data for treatment of hospitalized influenza patients with intravenous peramivir.
- The optimal duration and dosing of antiviral treatment are uncertain for severe or complicated influenza. Treatment regimens might need to be altered to fit the clinical circumstances.
- Decisions about extended (longer) duration of treatment should be guided by clinical judgment in patients whose illness is prolonged.
- Virologic testing of lower respiratory tract specimens by real-time reverse transcription-polymerase chain reaction (RT-PCR) can help guide decisions about extended treatment in hospitalized influenza patients with severe and prolonged illness. Critically ill patients with respiratory failure can have prolonged influenza viral replication in the lower respiratory tract and might benefit from longer duration of treatment.
- Longer treatment regimens might be necessary in immunocompromised patients who may have prolonged influenza viral replication. Such patients are at risk of emergence of influenza viruses with reduced susceptibility or antiviral resistance during or after antiviral treatment.
- A higher dose of oral or enterically administered oseltamivir has been recommended by some experts (e.g., 150 mg twice daily in adults with normal renal function) for treatment of influenza in immunocompromised patients and in severely ill hospitalized patients. However, oral or enterically administered oseltamivir at standard doses has been reported to be adequately absorbed in critically ill adults to therapeutic blood levels (Ariano, 2010), and available data suggest that higher dosing may not provide additional clinical benefit (Abdel-Ghafar, 2008; Ariano, 2010; Kumar, 2010; Lee, 2013; South East Asia Infectious Disease Clinical Research Network, 2013).
- Studies indicate that exposure to oseltamivir carboxylate (the active metabolite of oseltamivir) is similar between obese and non-obese subjects for both 75 mg and 150 mg doses given twice daily (Ariano, 2010; Jittamala, 2014; Pai, 2011; Thorne-Humphrey, 2011).
- Limited data suggest that oseltamivir administered orally or by oro/naso gastric tube is well absorbed in critically ill influenza patients, including those receiving continuous renal replacement therapy, and/or on extracorporeal membrane oxygenation (Ariano, 2010; Eyler, 2012a; Eyler, 2012b; Giraud, 2011; Kromdijk, 2013; Lemaitre, 2012; Mulla, 2013; Taylor, 2008).
- If a hospitalized patient treated with oseltamivir or peramivir manifests progressive lower respiratory symptoms, resistant virus should be considered. However, clinicians should note that failure to improve or clinical deterioration during oseltamivir or peramivir treatment is more likely to be related to the natural history of acute lung injury and inflammatory damage or onset of other complications (e.g., renal failure, septic shock, ventilator-associated pneumonia) than to emergence of oseltamivir or peramivir resistance.
- Careful attention to ventilator and fluid management and to the prevention and treatment of secondary bacterial pneumonia (e.g., S. pneumoniae, S. pyogenes, and S. aureus, including MRSA) also is critical for severely ill patients (Bautista, 2010; Finelli, 2008; Hageman, 2006; Harper, 2009; Mandell, 2007; Mauad, 2010; Shieh, 2010).
Recommended Dosage and Duration of Influenza Antiviral Medications for Treatment or Chemoprophylaxis
Table 2. Recommended Dosage and Duration of Influenza Antiviral Medications for Treatment or Chemoprophylaxis
Antiviral Agent
Use
Children
Oral Oseltamivir
Treatment (5 days)1
If younger than 1 yr old2: 3 mg/kg/dose twice daily3,4 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg twice a day >15 to 23 kg, the dose is 45 mg twice a day >23 to 40 kg, the dose is 60 mg twice a day >40 kg, the dose is 75 mg twice a day
Chemoprophylaxis (7 days)5
If child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless situation is judged critical due to limited data in this age group. If child is 3 months or older and younger than 1 yr old2 3 mg/ kg/dose once daily3 If 1 yr or older, dose varies by child’s weight: 15 kg or less, the dose is 30 mg once a day >15 to 23 kg, the dose is 45 mg once a day >23 to 40 kg, the dose is 60 mg once a day >40 kg, the dose is 75 mg once a day
Inhaled Zanamivir6
Treatment (5 days)
10 mg (two 5-mg inhalations) twice daily
(FDA approved and recommended for use in children 7 yrs or older)
Chemoprophylaxis (7 days)5
10 mg (two 5-mg inhalations) once daily
(FDA approved for and recommended for use in children 5 yrs or older)
Intravenous Peramivir7
Treatment (1 day)1
(6 months to 12 yrs of age) One 12 mg/kg dose, up to 600 mg maximum, via intravenous infusion for a minimum of 15 minutes
(FDA approved and recommended for use in children 6 months or older)
Chemoprophylaxis8
Not recommended
Oral Baloxavir9
Treatment (1 day)
(5 yrs and older weighing <20 kg: single dose of 2 mg/kg by suspension; weighing 20 kg to <80 kg: single dose of 40 mg by tablet or suspension; weighing ≥80 kg: single dose of 80 mg by tablet or suspension)9
FDA approved for use in children 5 years and older who are otherwise healthy or at high risk of developing influenza-related complications
(Baloxavir marboxil suspension is not currently available in the United States.)
Chemoprophylaxis9
FDA approved for post-exposure prophylaxis for persons aged 5 years and older. Dosage is the same as for treatment.
Abbreviations: N/A = not approved
- Longer treatment duration may be needed for severely ill patients. Dose adjustment is recommended for patients with reduced creatinine clearance. More information is available in the "Dosing in Adult Patients with Renal Impairment" section and Table 3.
- Oral oseltamivir is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset with twice-daily dosing in people 14 days and older, and for chemoprophylaxis with once-daily dosing in people 1 year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants less than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year of age, is recommended by CDC and the American Academy of Pediatrics (Recommendations for Prevention and Control of Influenza in Children, 2023–2024).
- This is the FDA-approved oral oseltamivir treatment dose for infants 14 days and older and less than 1 year old and provides oseltamivir exposure in children similar to that achieved by the approved dose of 75 mg orally twice daily for adults, as shown in two studies of oseltamivir pharmacokinetics in children (Kimberlin, 2013 [CASG 114], EU study WP2284, FDA Clinical Pharmacology Review). The American Academy of Pediatrics has recommended an oseltamivir treatment dose of 3.5 mg/kg orally twice daily for infants 9-11 months old, on the basis of data which indicated that a higher dose of 3.5 mg/kg was needed to achieve the protocol-defined targeted exposure for this cohort as defined in the CASG 114 study (Kimberlin, 2013). It is unknown whether this higher dose will improve efficacy or prevent the development of antiviral resistance. However, there is no evidence that the 3.5 mg/kg dose is harmful or causes more adverse events to infants in this age group.
- Current weight-based dosing recommendations are not appropriate for premature infants. Premature infants might have slower clearance of oral oseltamivir because of immature renal function, and doses recommended for full-term infants might lead to very high drug concentrations in this age group. CDC recommends dosing as also recommended by the Advisory Committee on Immunization Practices (ACIP) (Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP)-United States, 2024-25): limited data from the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group provide the basis for dosing preterm infants using their postmenstrual age (gestational age + chronological age): 1.0 mg/kg/dose, orally, twice daily, for those <38 weeks postmenstrual age; 1.5 mg/kg/dose, orally, twice daily, for those 38 through 40 weeks postmenstrual age; 3.0 mg/kg/dose, orally, twice daily, for those >40 weeks postmenstrual age.
- Special Considerations for Institutional Settings section for details regarding duration of chemoprophylaxis for outbreaks in institutional settings.
- Inhaled zanamivir is approved for treatment of acute uncomplicated influenza within 2 days of illness onset with twice-daily dosing in people aged ≥7 years, and for chemoprophylaxis with once-daily dosing in people aged ≥5 years.
- Intravenous peramivir is approved for treatment of acute uncomplicated influenza within 2 days of illness onset with a single dose in people aged ≥6 months. Daily dosing for a minimum of 5 days was used in clinical trials of hospitalized patients with influenza (de Jong, 2014, Ison, 2014). Dose adjustment is recommended for patients with reduced creatinine clearance. More information is available in the "Dosing in Adult Patients with Renal Impairment" section and Table 3.
- There are no data for use of peramivir for chemoprophylaxis of influenza.
- Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people aged ≥5 years who are otherwise healthy or at high risk of developing influenza-related complications. Baloxavir marboxil (Xofluza) [package insert]. Baloxavir marboxil suspension is not currently available in the United States. Baloxavir marboxil should not be administered with dairy products, calcium-fortified beverages, polyvalent cation-containing laxatives, antacids or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc); co-administration with polyvalent cation-containing products may decrease plasma concentrations of baloxavir which may reduce efficacy. There are no available published data from clinical trials for baloxavir treatment of influenza in non-hospitalized patients who are pregnant, immunocompromised, or have severe disease.
Influenza Antiviral Resistance Considerations
- Antiviral resistance and reduced susceptibility to the neuraminidase inhibitors and to baloxavir among circulating influenza viruses is currently very low, but this can change.
- Weekly surveillance data on susceptibility of circulating influenza viruses to antivirals in the U.S. this season, FluView Weekly U.S. Influenza Surveillance Report is available.
- Influenza viruses with reduced susceptibility or resistance to antivirals can occur sporadically (Hurt, 2011; Takashita, 2013; Takashita, 2014) or emerge during or after antiviral treatment in some patients (e.g., immunocompromised).
- Oseltamivir resistance in influenza A(H3N2) and A(H1N1)pdm09 viruses can develop during treatment, particularly in young children (Roosenhoff, 2019; Lina, 2018;), and immunocompromised persons (Memoli, 2014).
- Influenza viruses may become less susceptible or resistant to oseltamivir and peramivir during antiviral treatment with one of these drugs and remain susceptible to zanamivir; this has been reported most often for influenza A(H1N1)pdm09 viruses (Graitcer, 2011; Lackenby, 2011; Memoli, 2010; Nguyen, 2010; Nguyen, 2012).
- Influenza A(H1N1)pdm09 viruses have also emerged that are resistant to all neuraminidase inhibitors, including zanamivir, in highly immunosuppressed patients on prolonged neuraminidase inhibitor treatment (Tamura, 2015; L'Huillier, 2015).
- Human-to-human transmission of influenza A(H1N1)pdm09 viruses with an H275Y mutation in viral neuraminidase conferring resistance to oseltamivir has been reported among severely immunocompromised patients in hospital units, (Gooskens, 2009; Chen, 2011;) and in the community (Hibino, 2017; Le, 2008; Hurt, 2011; Hurt, 2012; Takashita, 2013), but currently appears to be uncommon.
- Following treatment with baloxavir, emergence of viruses with molecular markers associated with reduced susceptibility to baloxavir has been observed in clinical trials in immunocompetent children and adults, with higher detection among baloxavir-treated pediatric patients aged <12 years compared with adults (Hayden, 2018; Omoto, 2018; Hirostu, 2019; Uehara, 2019; Takashita, 2019).
- Limited human-to-human transmission of influenza A(H3N2) virus with reduced susceptibility to baloxavir has been reported sporadically in Japanese children (Takashita, 2019; Takashita 2019; Imai, 2019), but currently appears to be uncommon.
- Molecular analyses can detect genetic changes in influenza viruses associated with resistance and reduced susceptibility to oseltamivir and peramivir. The CDC Influenza Division is available for consultation regarding antiviral susceptibility testing as needed. Information about neuraminidase inhibitor susceptibility testing and interpretation of results of neuraminidase inhibition assays is available on the WHO website.
Antiviral Treatment Efficacy and Effectiveness
Patients with Uncomplicated Influenza
- Meta-analyses of randomized controlled clinical trials (RCTs) have demonstrated efficacy of early initiation of treatment (started within 36 to 48 hours of illness onset) with neuraminidase inhibitors in reducing duration of fever and illness symptoms compared with placebo in otherwise healthy children and adults with uncomplicated influenza ( Jefferson, 2014; Dobson, 2015; Malosh, 2018; Liu, 2021).
- One randomized clinical trial in children with uncomplicated influenza demonstrated a modest reduction in duration of symptoms and influenza virus shedding in patients initiating treatment after 48 hours; post hoc analysis suggested that oseltamivir treatment initiated 72 hours after illness onset reduced symptoms by one day compared with placebo (Fry, 2014).
- A meta-analysis of RCTs comparing early treatment with oseltamivir to placebo or nonactive controls among adults and adolescents with uncomplicated influenza found no reduction in the risk of subsequent hospitalization with influenza but was underpowered to detect an effect given the very low rate of hospitalization among the trial populations (Hanula 2023; Antoon 2023; Uyeki 2023).
- RCTs and a meta-analysis of RCTs comparing baloxavir to placebo or oseltamivir among children and adults with uncomplicated influenza found that baloxavir was superior to placebo and comparable to oseltamivir in reducing symptom duration (Kuo, 2021; Portsmouth 2021).
- There are no available data on the use of baloxavir for treatment of influenza more than 2 days after illness onset in outpatients.
Hospitalized Patients
- No completed, sufficiently powered, randomized, placebo-controlled clinical trials have been conducted of monotherapy with neuraminidase inhibitors for treatment of influenza in hospitalized patients; studies supporting the licensure of oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir were conducted in outpatients, primarily among previously healthy persons with uncomplicated illness.
- A secondary analysis of a multi-center unblinded clinical trial of oseltamivir treatment started within 24 hours of enrollment after hospital admission versus standard of care in adults hospitalized for lower respiratory tract infection reported that oseltamivir treatment lowered the risk of clinical failure in patients with laboratory-confirmed influenza; clinical failure was defined as failure to improve with 7 days, transfer to ICU care 24 hours after admission, or rehospitalization or death within 30 days (Wiemken, 2021).
- Several observational studies in hospitalized influenza patients have shown clinical benefit of neuraminidase inhibitor antiviral treatment compared with no treatment, particularly when started within two days of illness onset, or as soon as possible after hospital admission, including reducing the duration of hospitalization, and reducing the risk of ICU transfer, invasive mechanical ventilation or risk of death (Coffin, 2011; Hsu, 2012; Louie, 2013; Muthuri, 2013; Muthuri, 2014; Miyakawa, 2019; Lytras, 2019; Chen, 2020; Chen, 2020; Venkatesan, 2020; Katzen, 2019; Reacher, 2019; Walsh, 2022).
- Some observational studies have reported that oral oseltamivir treatment started 4 and 5 days after illness onset in patients hospitalized with suspected or confirmed influenza was associated with lower risk of death (EH Lee, 2010; N Lee, 2008; N Lee, 2010; Louie, 2012; McGeer, 2007), although one report found this benefit only in hospitalized adult patients in the ICU (Muthuri, 2014). A small number of observational studies and one meta-analysis of observational studies of hospitalized influenza patients reported that neuraminidase inhibitor treatment was not associated with a reduction in risk of death (Choi, 2017; Wolkewitz, 2016; Heneghan, 2016).
- One randomized clinical trial reported that combination neuraminidase inhibitor (primarily oseltamivir) and baloxavir treatment of hospitalized influenza patients aged ≥12 years did not result in superior clinical benefit (time to clinical improvement) compared to treatment with neuraminidase inhibitor and placebo, indicating that adding baloxavir did not result in additional clinical benefit, but significantly reduced nasopharyngeal influenza viral RNA levels (Kumar, 2022).
- Observational studies in hospitalized patients with influenza have reported that clinical benefit is greatest when oseltamivir is started within 48 hours of illness onset (Hsu, 2012; Louie, 2013; Muthuri, 2013; Muthuri, 2014). However, some studies suggest that antiviral treatment might still be beneficial in hospitalized patients when started up to 4 or 5 days after illness onset (Louie, 2012; Yu, 2011).
- Antiviral treatment during any trimester of pregnancy for influenza A(H1N1)pdm09 virus infection has been shown to be most beneficial in preventing respiratory failure and death when started within 2 days of illness (Siston, 2010).
- Observational studies in hospitalized patients with influenza have reported greater clinical benefit when oseltamivir or other neuraminidase inhibitor treatment are started at or promptly after hospital admission compared with later treatment initiation or no antiviral treatment (Katzen, 2018, Venkatesan, 2019).
- Observational studies in hospitalized adult patients with influenza have reported that starting oseltamivir treatment within 48 hours of hospital admission can reduce ICU admission, 30-day readmissions and mortality compared with no treatment or later initiation of treatment (Sharma 2021, Groeneveld 2020).
Duration of Treatment or Chemoprophylaxis
- Treatment: Recommended duration for antiviral treatment of uncomplicated influenza in outpatients is 5 days for oral oseltamivir or inhaled zanamivir. For treatment of uncomplicated influenza with intravenous peramivir or oral baloxavir, a single dose is recommended. Longer daily dosing (oral oseltamivir or intravenous peramivir) can be considered for hospitalized patients with influenza who remain severely ill after 5 days of treatment. Treatment should be started as soon as possible after symptom onset for the greatest clinical benefit.
- Chemoprophylaxis: Recommended duration is 7 days (after last known exposure). For control of outbreaks in institutional settings (e.g., long-term care facilities for older adults and children) and hospitals, CDC recommends antiviral chemoprophylaxis of exposed residents with oral oseltamivir or inhaled zanamivir for a minimum of 2 weeks and continuing up to 1 week after the last known case was identified. Antiviral chemoprophylaxis is recommended for all residents, including those who have received influenza vaccination. For control of some institutional influenza outbreaks, post-exposure antiviral treatment has been used (e.g., oseltamivir twice daily for 5 days) instead of post-exposure antiviral chemoprophylaxis (Uyeki, 2019). Baloxavir is approved for post-exposure prophylaxis (single dose) of influenza in persons aged 5 years and older within 48 hours of contact with an individual with influenza.
Dosing in Adult Patients with Renal Impairment
Dose adjustment of oseltamivir is recommended for patients with creatinine clearance between 10 and 60 mL/min and patients with end-stage renal disease (ESRD) undergoing hemodialysis or continuous peritoneal dialysis receiving oseltamivir for the treatment or chemoprophylaxis of influenza. Oseltamivir is not recommended for patients with ESRD not undergoing dialysis. The recommended doses are detailed in Table 3; duration of treatment and chemoprophylaxis is the same as recommended for patients with normal renal function. The dose of intravenous peramivir should be reduced for patients with baseline creatinine clearance below 50 mL/min.
No dose adjustment is recommended for inhaled zanamivir for a 5-day course of treatment for patients with renal impairment. Pharmacokinetic analysis did not identify a clinically meaningful effect of renal function on the pharmacokinetics of baloxavir in patients with creatinine clearance 50 mL/min and above. The effects of severe renal impairment on the pharmacokinetics of baloxavir marboxil or its active metabolite, baloxavir, have not been evaluated.
Table 3. Recommended Oseltamivir and Peramivir Dose Adjustments for Treatment
Creatinine Clearance
Recommended Treatment Regimen
Oral Oseltamivir1
Creatinine clearance 61 to 90 mL/min
75 mg twice a day
Creatinine clearance 31 to 60 mL/min
30 mg twice a day
Creatinine clearance 11 to 30 mL/min
30 mg once daily
ESRD Patients on Hemodialysis Creatinine clearance ≤10 mL/min
30 mg after every hemodialysis cycle. Treatment duration not to exceed 5 days2
ESRD Patients on Continuous Ambulatory Peritoneal Dialysis4 Creatinine clearance ≤10 mL/min
A single 30 mg dose administered immediately after a dialysis exchange
Intravenous Peramivir (single dose)5
Creatinine clearance ≥50 mL/min
600 mg
Creatinine clearance 30 to 49 mL/min
200 mg
Creatinine clearance 10 to 29 mL/min
100 mg
ESRD Patients on Hemodialysis
Dose administered after dialysis at a dose adjusted based on creatinine clearance
Abbreviations: N/A = approved, not recommended
- Renal dosing of oseltamivir is not available in the package insert for pediatric patients. However, these tables may be useful for children who qualify for adult doses based on weight >40 kg.
- Assuming 3 hemodialysis sessions are performed in the 5- day period. Treatment can be initiated immediately if influenza symptoms develop during the 48 hours between hemodialysis sessions; however, the post-hemodialysis dose should still be administered independently of time of administration of the initial dose.
- An initial dose can be administered prior to the start of dialysis.
- Data derived from studies in continuous ambulatory peritoneal dialysis (CAPD) patients.
- Renal dosing from peramivir package insert is available for pediatric patients: Creatinine clearance ≥50 mL/min: 12 mg/kg (up to maximum dose of 600 mg); Creatinine clearance 30 to 49 mL/min: 4 mg/kg; Creatinine clearance 10 to 29 mL/min: 2 mg/kg.
Adverse Events
- When considering use of influenza antiviral medications, clinicians must consider the patient's age, weight and renal function; presence of other medical conditions; indications for use (i.e., chemoprophylaxis or therapy); and the potential for interaction with other medications.
- Some RCTs and meta-analyses of RCTs have indicated that gastrointestinal symptoms such as nausea and vomiting are increased with oral oseltamivir compared with placebo; these adverse events may be less likely when oseltamivir is taken with food (Aoki, 2003; Malosh, 2018; Dobson, 2015, Liu, 2021). An RCT of empiric oseltamivir treatment compared to placebo in children 0-9 years of age hospitalized with acute respiratory illness, including >300 infants <1 year of age, found no difference in rates of vomiting or other gastrointestinal symptoms (Dawood, 2016).
- For more information on safety, effectiveness and dosing for oral oseltamivir, inhaled zanamivir, intravenous peramivir, or oral baloxavir, visit Antiviral Drugs or consult the package inserts.
Drug Interactions
- Co-administration of baloxavir with polyvalent cation-containing products may decrease plasma concentrations of baloxavir which may reduce efficacy. Avoid co-administration of baloxavir with polyvalent cation-containing laxatives, antacids, or oral supplements (e.g., calcium, iron, magnesium, selenium, or zinc).
- Concurrent administration of antiviral drugs with intranasal live attenuated influenza vaccine (LAIV) may inhibit viral replication of LAIV and thereby decrease the effectiveness of LAIV vaccination. LAIV should not be given if oseltamivir or zanamivir was administered within 48 hours of planned vaccination, or if peramivir was administered within 5 days of planned vaccination, or if baloxavir was administered within 17 days of planned vaccination. If LAIV is given, and antiviral medications are subsequently administered up to two weeks after vaccination, the effectiveness of LAIV might be reduced, and persons who receive these antiviral medications within two weeks after receiving LAIV should be revaccinated with another appropriate influenza vaccine (e.g., IIV or RIV4).
Seasonal Influenza (Flu) site, CDC-INFO, or CDC at 800-CDC-INFO (English and Spanish) or 888-232-6348 (TTY) are available for more information.
- Oral or enterically administered oseltamivir is the recommended antiviral for patients with severe, complicated, or progressive illness who are not hospitalized, and for hospitalized influenza patients. For hospitalized patients who cannot tolerate or absorb oral or enterically administered oseltamivir because of suspected or known gastric stasis, malabsorption, or gastrointestinal bleeding, intravenous peramivir may be considered (Lee, 2017; de Jong, 2014; Ison, 2014; Ison, 2013). There are insufficient data to support general use of inhaled zanamivir and intravenous peramivir in patients with severe influenza disease. There are no available data from clinical trials on use of baloxavir treatment in patients with severe influenza disease who are not hospitalized. Oral oseltamivir and oral baloxavir are available treatment options for patients at higher risk for influenza complication depending upon their underlying conditions and age (Table 1). Data on use of peramivir or zanamivir are very limited in high-risk outpatients with influenza.
