At a glance
- CDC cannot accept any sample for viral hemorrhagic fever (VHF) or other high-consequence disease testing without prior consultation.
- Specimens being submitted for testing for VHFs and other high-consequence diseases must be packed and shipped correctly to prevent possible exposures and for the safety of those handling the packages.
Scope of guidance
This guidance applies to viral hemorrhagic fevers caused by infections with:
- Filoviruses (ebolaviruses and marburgviruses),
- Arenaviruses [Lassa, Lujo, and South American hemorrhagic fever viruses (Guanarito virus, Sabia virus, Junin virus, Chapare virus, Machupo virus)],
- Rift Valley fever virus, and
- Crimean Congo hemorrhagic fever virus.
This guidance also applies to other high-consequence diseases that require a specialized laboratory, are highly pathogenic, and have no vaccine or treatment currently available, like Nipah virus disease.
Specimen acceptance criteria
- You must consult with your state and local public health officials prior to testing patients for a VHF or other high-consequence disease is required [24-hour Epi On Call contact list]. They will coordinate a consultation with CDC to determine whether the patient meets the criteria for testing as a suspect VHF or other high-consequence disease case. These criteria include:
- Compatible symptoms including measured (≥100.4°F/38.0°C) or subjective fever, severe headache, fatigue, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage AND
- An epidemiological risk factor within the incubation period of the VHF or other high-consequence virus preceding symptom onset
- Compatible symptoms including measured (≥100.4°F/38.0°C) or subjective fever, severe headache, fatigue, muscle pain, vomiting, diarrhea, abdominal pain, or unexplained hemorrhage AND
- The United States Occupational Safety and Health Administration (OSHA) developed Bloodborne Pathogens Standard (29 CFR 1910.1030) to reduce the potential exposure of healthcare and lab personnel to all bloodborne pathogens. Employees of all U.S. laboratories handling patient specimens must always comply with this standard. Strict adherence is an initial step to protecting personnel.
- Prior to receiving specimens, the laboratory director, safety officer, and/or other responsible people should already have a site-specific risk assessment to minimize risk to lab personnel from potential exposure from sprays, splashes, or aerosols generated during laboratory activities. Risks should be mitigated by implementing engineering controls, administrative and work practice controls, and use of appropriate personal protective equipment (PPE). A plan for appropriate waste management should also exist and be implemented.
- Most VHFs or other high-consequence diseases are Department of Transportation (DOT)-classified as Category A infectious substances.
- Specimens from patients with a presumptive or suspected VHF or other high-consequence diseases should also be packaged and shipped as Category A infectious substances
- Specimens from patients with a presumptive or suspected VHF or other high-consequence diseases should also be packaged and shipped as Category A infectious substances
- Immediately report potential exposures to blood, body fluids, or other infectious materials according to your institution's policies and procedures.
Forms to include
Once testing is approved following consultation with CDC, please complete and submit the CDC 50.34 Specimen Submission form along with the specimen you are requesting to test.
To submit a non-human primate specimen for testing, complete the Primate Testing Form [PDF – 2 pages], following the instructions included on the form. Include a copy of the form with the specimen, saving another copy for your records. Email spather@cdc.gov for questions.
Packaging and labeling guidelines
- Category A substances like specimens that are presumptive positive or suspected for VHFs and other high-consequence diseases should be packaged and shipped in accordance with the DOT's Hazardous Materials Regulations (HMR) Title 49 Code of Federal Regulations (CFR) 173.196. Shipping regulations may change over time, consult certified Dangerous Goods shipping experts and CDC for current requirements.
- All people involved in packaging and shipping potentially infectious substances should be trained and certified in compliance with DOT or the International Air Transport Association (IATA) requirements every two years.
- Do not open collection tubes while packaging specimens to be shipped for VHF or high-consequence disease testing. Opening tubes will break the vacuum seal and increase the risk of leakage during transport, exposing the packer to the contents.
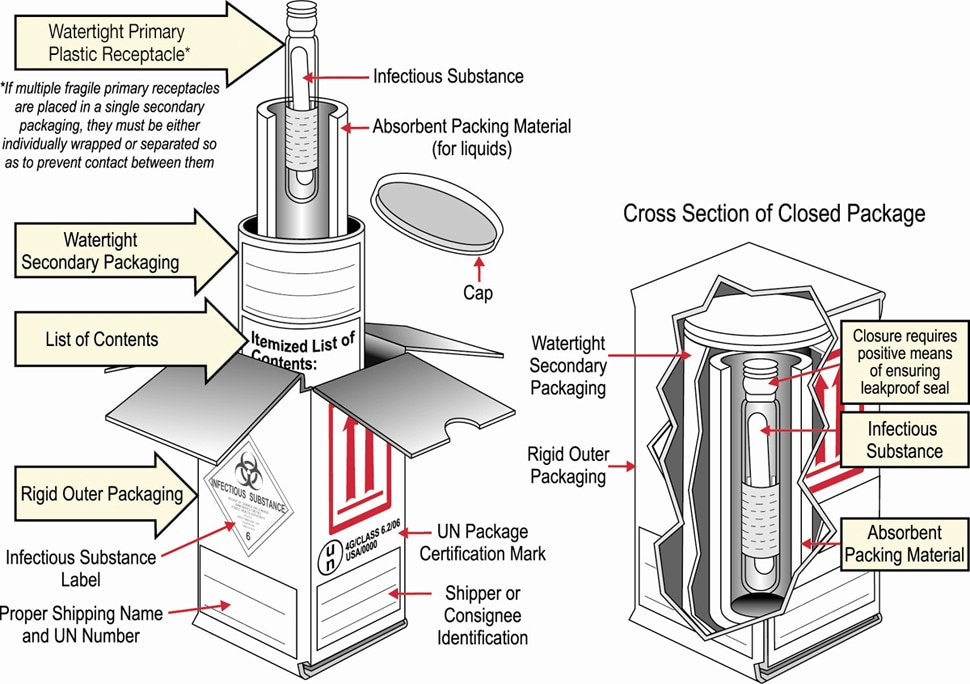
- Package specimens following the triple packaging system as shown in the diagram below, which consists of (1) a primary container (a sealable specimen container) wrapped with absorbent material, (2) a secondary container (watertight, leak-proof), and (3) an outer shipping package that meet Category A shipping requirements.
- For questions about (packaging) transportation regulations, contact the U.S. DOT Hazardous Materials Information Center at 1-800-467-4922.
- Coordinate with staff at the laboratory that will be receiving the specimens on appropriate transport conditions. For example:
- Laboratory Response Network laboratories require specimens be sent on cold packs.
- CDC requires specimens be sent frozen on dry ice.
- Laboratory Response Network laboratories require specimens be sent on cold packs.
- CDC will not accept specimens without prior consultation and approval. CDC's Viral Special Pathogens Branch (VSPB) is available 24/7 for consultations by calling the CDC Emergency Operations Center at 770-488-7100 and requesting VSPB's on-call epidemiologist.
Specimen shipping
After testing at CDC is approved, email tracking number to spather@cdc.gov.
Package in accordance with the International Air Transport Association (I.A.T.A.) regulations to prevent leakage.
Ship to:
Centers for Disease Control and Prevention
RDSB/STAT Unit 70
1600 Clifton Road, NE
Atlanta, GA 30329
Specify specimen storage requirement (Frozen) on the outside of the box. Label sample as diagnostic specimen and include the following information:
- Your name
- Patient's name
- Test(s) requested
- Date of collection
- Laboratory or accession number
- Type of specimen being shipped
- Fill out and include the CDC Form 50.34
- If required, the Primate Submission form [PDF – 2 pages] or the Hantavirus Disease Case Report form [PDF – 1 page] (for hantavirus testing only).
- Send specimens by overnight courier (FedEx preferred). Please request early delivery to expedite accessioning and testing. Fax or email airway bill number and packing list to VSPB at (404) 471-2526. For international submitters, consider direct shipment with an airline to expedite arrival at CDC. Do not ship for weekend delivery unless instructed by CDC.
- Subsequent shipments during outbreak may require U.S. Public Health permits.
