At a glance

Summary
The Advisory Committee on Immunization Practices (ACIP) recommends that all infants be protected from severe respiratory syncytial virus (RSV) disease by one of two immunization options: a monoclonal antibody (nirsevimab) for infants or maternal vaccine.12 Most infants do not need both. Clinicians play a critical role in improving immunization uptake by making strong recommendations for immunization products, effectively communicating vaccine safety and effectiveness, and providing vaccines on site at their practice.34
To inform strategies for increasing nirsevimab and maternal RSV vaccine uptake during the 2024-2025 respiratory virus season, two surveys – one of pediatricians and the other of obstetrician/ gynecologists (OB/GYNs) – were conducted in October 2024. Both surveys assessed provider attitudes, immunization practices, and challenges related to administration of nirsevimab and maternal RSV vaccine.
Most pediatricians (77.0%) had ever offered nirsevimab; 63.0% of OB/GYNs offered RSV vaccine to pregnant women in their practice. Most providers also expressed high confidence in the safety and effectiveness of nirsevimab and maternal RSV vaccine. The main challenges for both pediatric and OB/GYN providers included the following: parent/caregiver or patient concerns about safety and effectiveness; the financial burden of purchasing the products and reimbursement. An additional challenge for pediatric providers was knowing maternal vaccination status to determine eligibility for nirsevimab administration; OB/GYN providers reported logistical issues around vaccine storage. Pediatricians in practices that never provided nirsevimab, as compared to those that did, more commonly reported financial burden of purchasing nirsevimab (45.7% vs. 26.0%). OB/GYNs that were not providing maternal RSV vaccine, as compared to those that did, more commonly reported cost and reimbursement issues (68.9% vs. 32.5%).
Methods
Two Porter Novelli View Health Care Practitioner surveys were conducted from October 2-10, 2024, among 200 U.S. pediatricians who reported offering at least some routine pediatric vaccines to patients and 200 U.S. OB/GYNs who reported providing care for pregnant women. The surveys were conducted by M3 Global Research.5 This panel is a market research opt-in panel; panelists are verified using a double opt-in sign up process with telephone confirmation at their place of employment. Panel members are provided with incentives for completing surveys based on the length and complexity of the survey and, provider specialty. Topics covered by the survey included 1) recommendation and offer of nirsevimab and maternal RSV vaccine; 2) attitudes about nirsevimab and maternal RSV vaccine; and 3) challenges in offering nirsevimab and maternal RSV vaccine. Respondents were asked to answer all questions considering the practice location where they spent most of their working time (i.e., primary worksite, if they worked at more than one practice location). Statistical comparisons were made using chi-squared tests in SAS (version 9.4; Cary, NC); a p-value of <0.05 was considered statistically significant.
Results
Infant nirsevimab administration
About three-quarters (77.0%) of pediatricians reported their practice had ever offered nirsevimab; 15.0% reported their practice had never offered nirsevimab, but planned to offer it for the 2024-25 respiratory virus season; 8.0% reported their practice had never offered nirsevimab, and did not plan to offer it for the 2024-25 season.
Over 95% of pediatricians agreed that nirsevimab is safe for infants and effective against severe disease in infants (Figure 1). Most pediatricians reported feeling confident discussing nirsevimab with parents/caregivers and making recommendation for its use.
The top reported challenges experienced or anticipated in offering nirsevimab included the following:
- parent/caregiver concerns around nirsevimab safety (44.0%),
- challenges knowing maternal RSV vaccination status to determine infant eligibility (33.5%),
- financial burden associated with purchasing nirsevimab (30.5%),
- challenges with reimbursement from private health insurance plans (30.5%) (Figure 2).
While not one of the top challenges, knowing whether an infant received nirsevimab at a birthing facility to determine eligibility for nirsevimab at pediatrician's office was reported by 20.0% of pediatricians.
Pediatricians whose practice had ever offered nirsevimab were more likely than pediatricians from practices that never offered nirsevimab to report challenges knowing maternal RSV vaccination status to determine infant eligibility and lack of demand from parents/caregivers as a challenge. In contrast, pediatricians whose practice had never offered nirsevimab were more likely than those from practices that ever offered to report financial burden in purchasing nirsevimab.
Figure 1. Pediatrician attitudes about nirsevimab, Pediatrician survey, October 2024, n=200
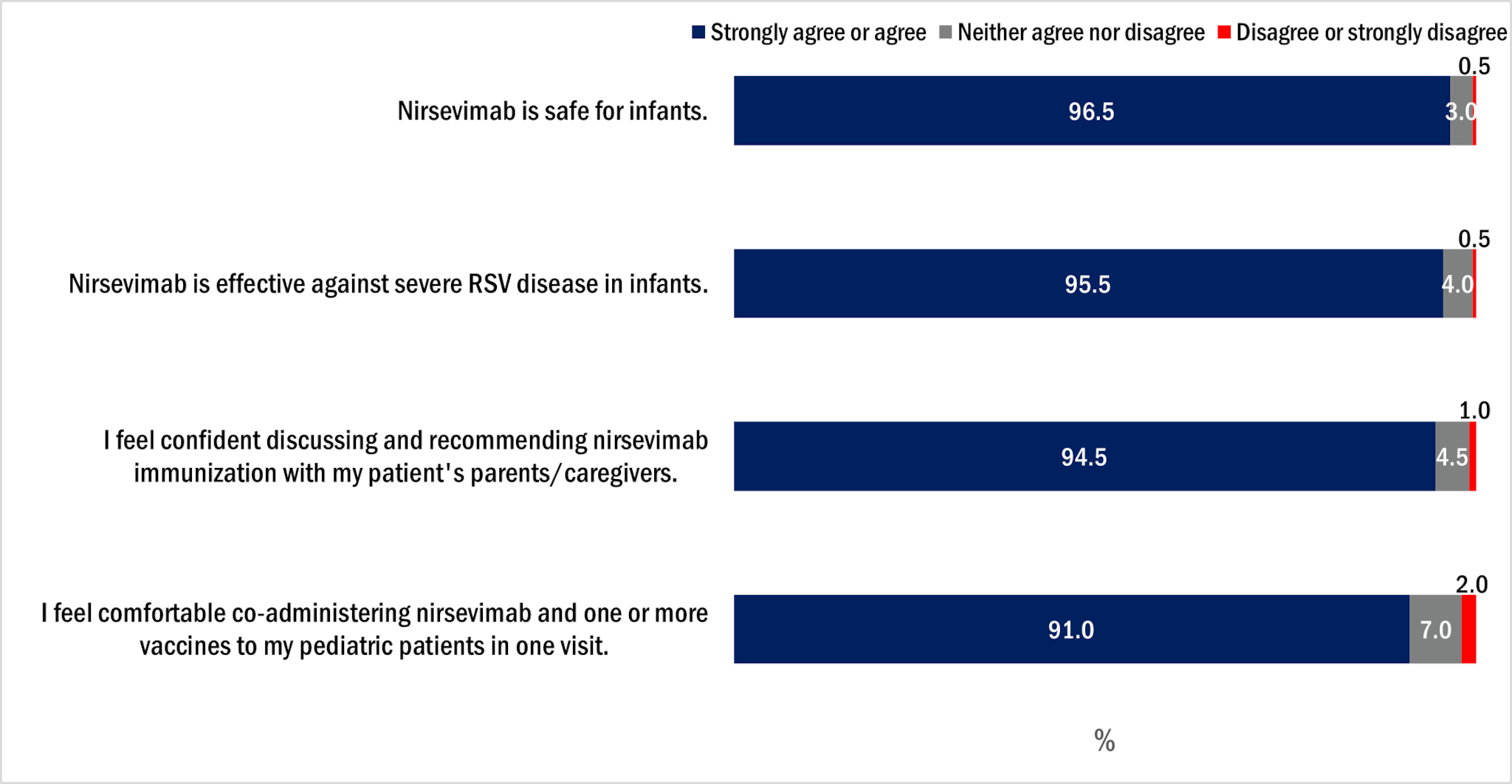
Figure 2. Frequency of main challenges* pediatricians reported experiencing or anticipated experiencing in offering nirsevimab, Pediatrician survey, October 2024, n=200
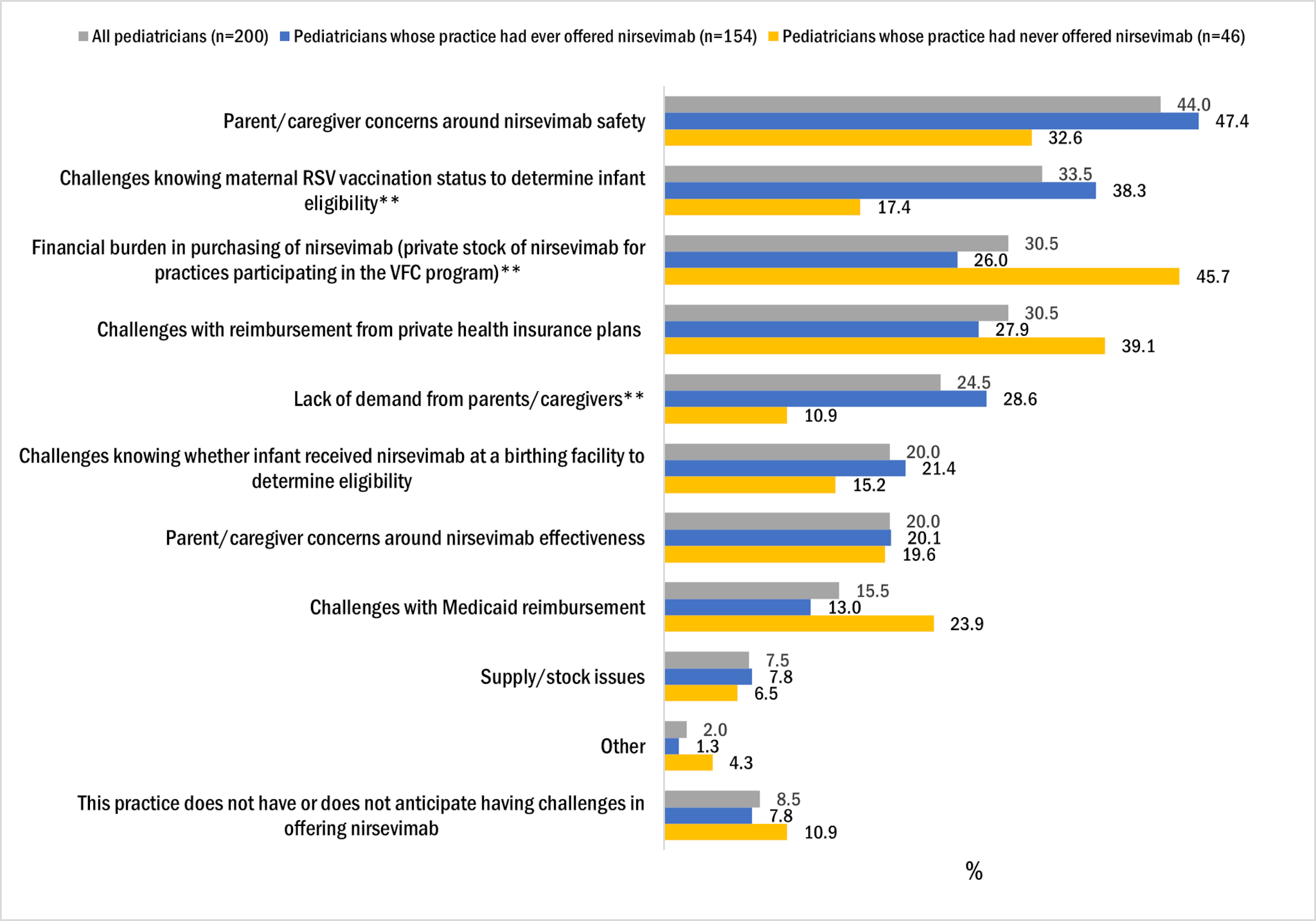
*Respondents were instructed to select up to 3 response categories.
**Proportion of pediatricians reporting challenge statistically significantly different between those whose practice had ever offered and those whose practice had never offered nirsevimab at p<0.05.
Maternal RSV vaccination
Most (87%) OB/GYNs reported that they recommended RSV vaccination and 63.0% reported that their practice offered RSV vaccination to pregnant women.
Over 90% of OB/GYNs agreed that RSV vaccination during pregnancy is safe and effective against severe disease in infants (Figure 3). A smaller proportion of OB/GYNs (75.0%) reported that they feel confident discussing both RSV vaccination during pregnancy and infant nirsevimab immunization.
The top three challenges OB/GYNs reported with offering maternal RSV vaccination were
- patient concerns around RSV vaccine safety (65.5%)
- cost and reimbursement issues (46.0%)
- patient concerns around vaccine effectiveness (28.0%) (Figure 4).
OB/GYNs whose practice offered RSV vaccine to pregnant women were more likely than those whose practice did not offer RSV vaccine to report patient concerns around vaccine safety and effectiveness. OB/GYNs whose practice did not offer RSV vaccine to pregnant women were more likely than those in practices that did offer RSV vaccine to report cost and reimbursement issues and logistical challenges for vaccine storage.
Figure 3. OB/GYN attitudes about maternal RSV vaccination, OB/GYN survey, October 2024, n=200
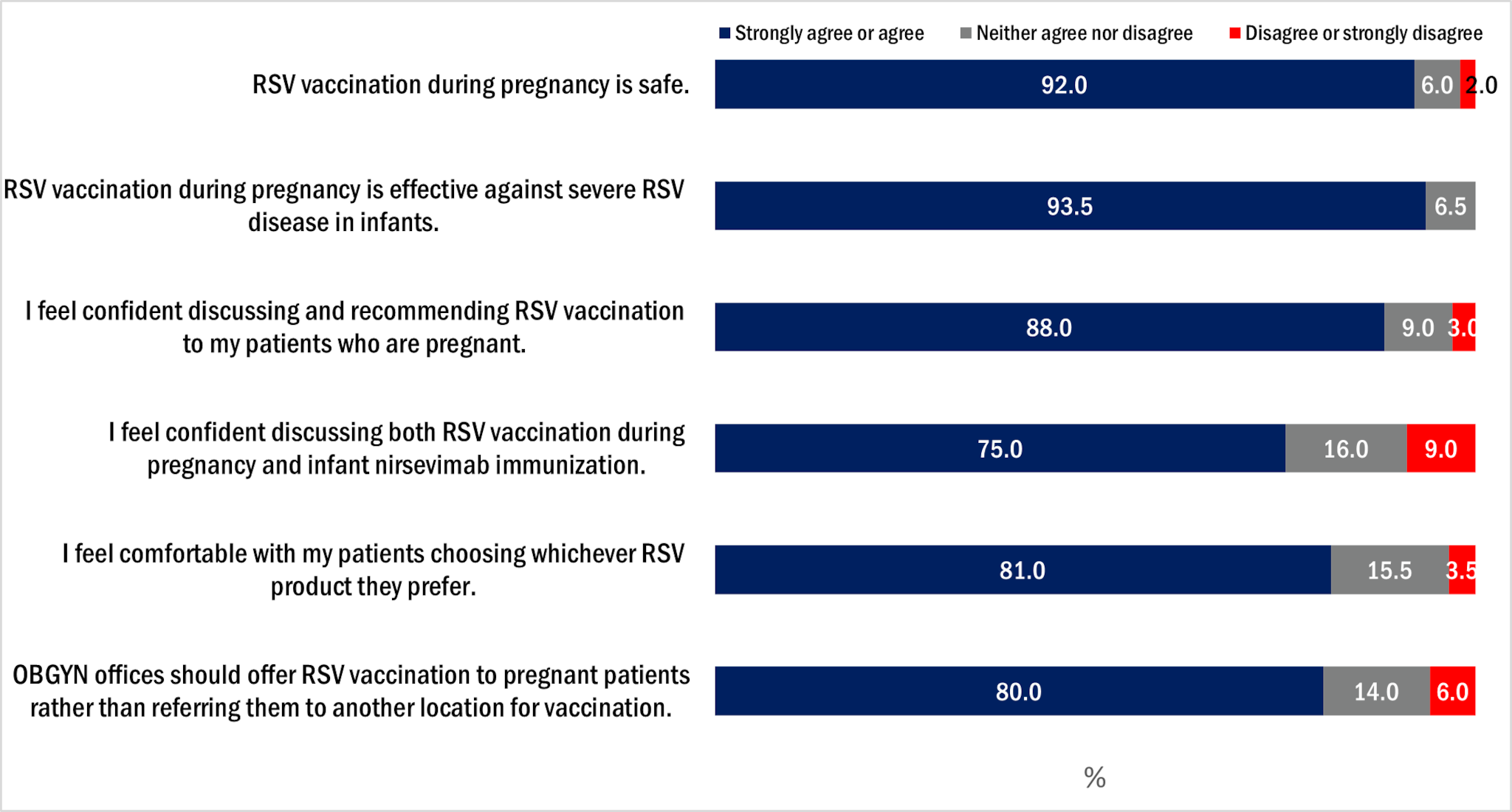
Figure 4. Frequency of main challenges* OB/GYNs reported in offering maternal RSV vaccination, OB/GYN survey, October 2024, n=200
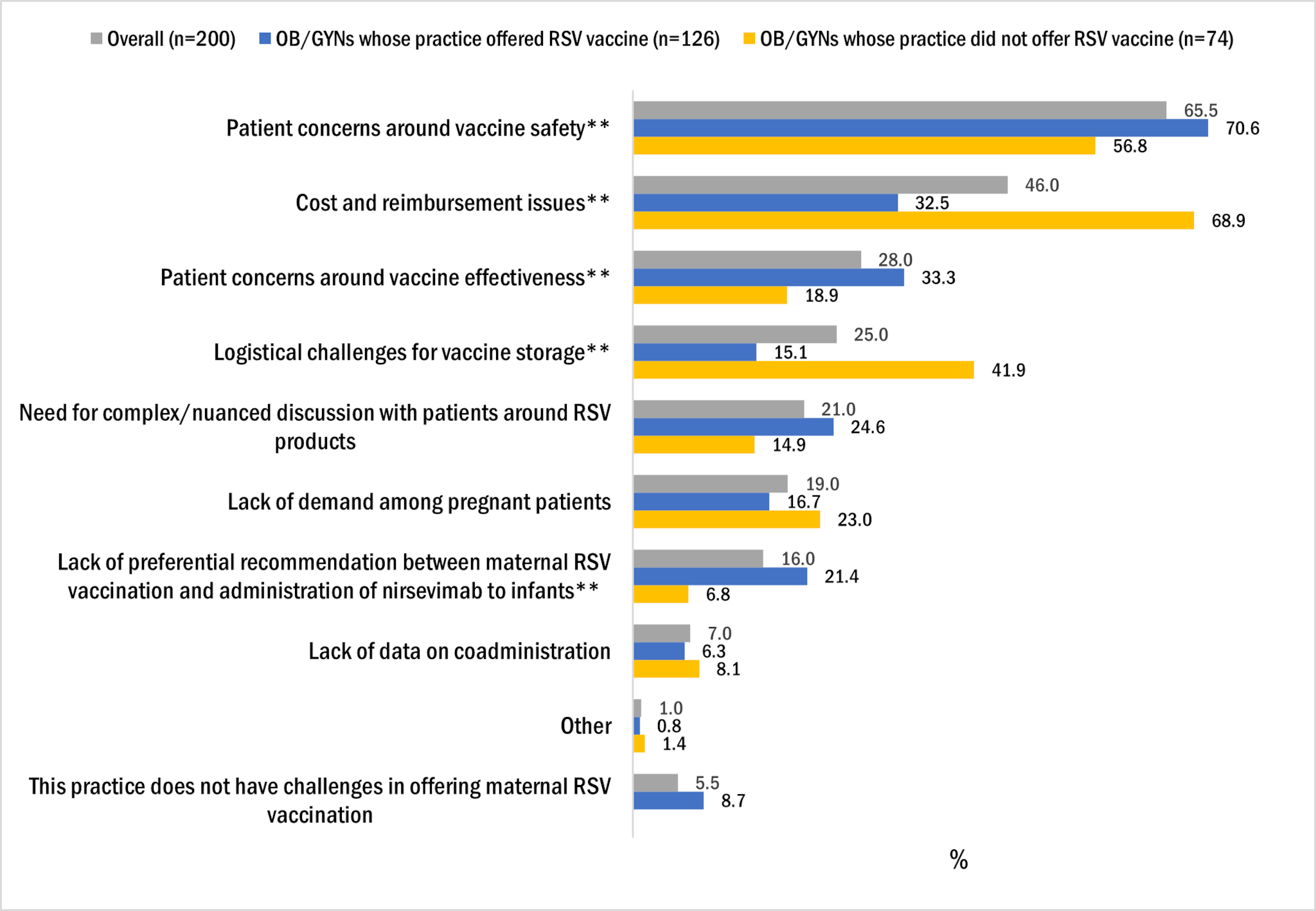
*Respondents were instructed to select up to 3 response categories.
**Proportion of OB/GYNs reporting challenge statistically significantly different between those whose practice has offered RSV vaccine and those whose practice has never offered RSV vaccine at p<0.05
Discussion
RSV is the leading cause of infant hospitalization in the U.S.6 There is a need to improve nirsevimab and maternal RSV vaccine uptake. Only 56% of infants were protected against severe RSV disease by either RSV product or both according to a survey conducted during March-April 2024 among pregnant women.7 The 2023-2024 RSV season was the first season during which nirsevimab and maternal RSV vaccine were recommended to protect infants from RSV disease. Challenges experienced during this initial season included timing of recommendations, limited availability of and familiarity with the products, and cost and reimbursement issues, all of which may have affected uptake of RSV immunization products.7 In this survey, physicians reported a number of challenges in offering RSV immunization products, some of which may reflect experiences unique to the 2023-2024 RSV season. This section highlights ways in which some of the reported physician challenges may be addressed and available resources for health care practitioners to increase nirsevimab and maternal RSV vaccine access and uptake.
First, online resources, job aids, and educational videos are available to support health care practitioners in communicating with parents/caregivers and patients about nirsevimab and maternal RSV vaccine, and how to effectively address questions on vaccine safety from parents/caregivers and pregnant women, including listening to patient questions with empathy to build trust. Clinicians remain the most trusted source for vaccine information and health care practitioner offer or referral for immunization is associated with higher uptake of immunization products.34 [Resources for health care practitioners] Medicaid providers can now bill for pediatric vaccine counseling even if the patient does not actually receive vaccination after counseling.8 A stand-alone vaccine counseling code can be used when a patient and/or caregiver receives counseling about a vaccine from a health care practitioner, but the patient does not actually receive the vaccine dose at the same time as the counseling (i.e., there is no actual delivery or injection of a vaccine during the practitioner visit). This applies to all recommended pediatric vaccines and includes nirsevimab. Clinicians are encouraged to continue the conversation at the patient's next visit, restating their strong recommendation and addressing patient concerns.
Second, clinicians and provider staff should document all immunization records in electronic health records (EHR) and immunization information systems (IISs) and use these systems to assess patient immunization status so that providers can assess maternal RSV vaccination status and whether an infant received nirsevimab at a birthing facility to determine infant eligibility for nirsevimab administration. Timely documentation may facilitate care coordination across OB/GYN offices, birthing hospitals, and pediatrician offices to ensure infants are protected against RSV disease. Reporting of immunization doses administered in IISs is a best practice and may be required in some jurisdictions.91011
Third, most private health insurance plans cover nirsevimab and maternal RSV vaccine administration, although some insurance plans' coverage may not begin until January 2025. Most people with Medicaid and Children's Health Insurance Program (CHIP) are guaranteed coverage of all vaccines recommended by the ACIP at no cost to them. Nirsevimab and maternal RSV vaccine, for those 18 years of age or younger and meet eligibility, are also covered by the VFC program.12 The VFC program is a federally funded program that provides vaccines at no cost to eligible children who might not otherwise be vaccinated because of inability to pay. It is expected that reimbursement during the 2024-2025 respiratory virus season should improve compared to last year.
Purchasing of nirsevimab and RSV vaccine can be financially burdensome, especially for small practices. Practices may reach out to manufacturers and wholesalers to inquire about financing options. CDC has updated VFC program policy flexibilities, allowing VFC providers temporary flexibility during which they will not be required to meet the private inventory requirement for nirsevimab until August 31, 2025, at the discretion of jurisdiction immunization program.13 Practices participating in the VFC program should reach out to their jurisdiction immunization program to discuss what may be allowed in their jurisdiction.
Limitations
The data were collected from opt-in internet panel surveys in which physicians agreed to participate and complete surveys; therefore, findings may not be generalizable to all pediatricians and OB/GYNs in the U.S and may be subject to sampling error, coverage error, and error associated with nonresponse. The survey was fielded among a small number of pediatricians and OB/GYNs during a short period of time. Few pediatricians and OB/GYNs with practices in rural areas participated in the surveys; therefore, provider attitudes and challenges unique to service provision in rural settings may be underrepresented in this report.
The pediatrician survey asked about the challenges experienced in the past and anticipated experiencing with offering of nirsevimab in one question; some responses, therefore, may be based on their experience from the previous respiratory virus season when nirsevimab and RSV vaccine were first recommended. The OB/GYN survey included a combined response category for cost and reimbursement issues, thus it was not possible to assess whether one is perceived to be a greater challenge than the other.
Authors
Yoonjae Kang, MPH1; Fan Zhang, MD1; Tara M Vogt, PhD, MPH1
1. Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA
Resources for health care practitioners
Health care provider RSV immunization resources:
- RSV Immunization Guidance for Infants and Young Children | RSV | CDC
- Infant RSV Prevention At-A-Glance | CDC
Respiratory Illnesses: Information for Health Care Providers | Respiratory Illnesses | CDC, including:
- Best Practices for Patient Care | Respiratory Illnesses | CDC
- Talking with Patients About Respiratory Virus Season | Respiratory Illnesses | CDC
References and Resources | RSV | CDC, including:
Easy-to-read immunization schedules:
- 2024 Recommended Immunizations for Birth Through 6 Years Old
- 2024 Recommended Immunizations for Adults Aged 19 Years and Older
The American College of Obstetricians and Gynecologists. Physician Tools. https://www.acog.org/programs/immunization-for-women/physician-tools
- Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–925. DOI: http://dx.doi.org/10.15585/mmwr.mm7234a4
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–1122. DOI: http://dx.doi.org/10.15585/mmwr.mm7241e1
- Lindley MC, Kahn KE, Bardenheier BH, et al. Vital Signs: Burden and Prevention of Influenza and Pertussis Among Pregnant Women and Infants — United States. MMWR Morb Mortal Wkly Rep 2019;68:885–892. DOI: http://dx.doi.org/10.15585/mmwr.mm6840e1
- Flu, Tdap, and COVID-19 Vaccination Coverage Among Pregnant Women – United States, April 2024. https://www.cdc.gov/fluvaxview/coverage-by-season/pregnant-april-2024.html
- M3 Global Research. Accessed November 4, 2024. https://m3globalresearch.blog/about-m3/
- Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009–2019: a study of the National (Nationwide) Inpatient Sample. J Infect Dis 2022;226(Suppl 2):S154–63. https://doi.org/10.1093/infdis/jiac120
- Razzaghi H, Garacci E, Kahn KE, et al. Maternal Respiratory Syncytial Virus Vaccination and Receipt of Respiratory Syncytial Virus Antibody (Nirsevimab) by Infants Aged <8 Months — United States, April 2024. MMWR Morb Mortal Wkly Rep 2024;73:837–843. DOI: http://dx.doi.org/10.15585/mmwr.mm7338a2
- Centers for Medicare & Medicaid Services. Coverage and Payment of Vaccines and Vaccine Administration under Medicaid, the Children's Health Insurance Program, and Basic Health Program. https://www.medicaid.gov/medicaid/quality-of-care/downloads/vacines-coverage-payment.pdf.
- Centers for Disease Control and Prevention: Vaccine Information for Adults. Adult Immunization Standards. https://www.cdc.gov/vaccines-adults/hcp/imz-standards/index.html
- Centers for Disease Control and Prevention: VFC Operations Guide. https://www.cdc.gov/vaccines-for-children/media/pdfs/2024/08/vfc-ops-guide_version-4.0_july-2024_low-res-508-rev-2.pdf.
- Centers for Disease Control and Prevention: Immunization Information Systems (IIS). IIS Policy and Legislation. https://www.cdc.gov/iis/policy-legislation/index.html
- Centers for Disease Control and Prevention: Vaccines for Children Program (VFC). Vaccines for Children (VFC) Program Eligibility. https://www.cdc.gov/vaccines-for-children/hcp/program-eligibility/index.html
- CDC's Vaccines for Children Program Addendum: Special Considerations for COVID-19 and Nirsevimab. Updated March 29, 2024. https://www.cdc.gov/vaccines-for-children/media/pdfs/2024/08/operations-guide-covid-19-addendum-2024-4-2_002-508.pdf.
