What to know
- CDC recommends all babies be protected from severe RSV by one of two immunization options: A maternal RSV vaccine given to the mother during pregnancy or an RSV antibody given to your baby. Most babies do not need both.
- The maternal RSV vaccine (Pfizer's Abrysvo) is given during weeks 32-36 of pregnancy. Maternal antibodies protect the baby against RSV for approximately 6 months after birth.
- A long-acting infant RSV antibody (nirsevimab or clesrovimab) can be given to babies. This antibody provides immediate protection against RSV and lasts at least 5 months.
- Some young children may also be eligible for nirsevimab.

Overview
Maternal RSV vaccine (Pfizer's Abrysvo)
The maternal RSV vaccine (Pfizer’s Abrysvo) is recommended if you are 32-36 weeks pregnant during September through January in most of the U.S. Like other vaccines, the RSV vaccine teaches your immune system to fight disease. Even though you are not at high risk for severe RSV, this vaccine is important because you will pass the protection to your baby. From the time you are vaccinated, it takes about 2 weeks to develop protection (antibodies) and for protection to pass on to your baby.
Protection will last during your baby’s first 6 months, while they are at highest risk of severe RSV.
If you have already received a maternal RSV vaccine during any previous pregnancy, CDC does not currently recommend you get another dose of RSV vaccine when you are pregnant again. Instead, your baby should receive a long-acting RSV antibody after birth.
Infant RSV antibodies
An infant RSV antibody is recommended for all babies younger than 8 months of age born to mothers who did not receive a maternal RSV vaccine (Pfizer’s Abrysvo) during pregnancy. Two long-acting RSV antibodies are available for infants — nirsevimab or clesrovimab. An infant RSV antibody dose should be given to babies shortly before the RSV season, or within 1 week after birth if born during October through March in most of the U.S.
Nirsevimab is also recommended for a small group of young children 8-19 months of age who are at increased risk for severe RSV. Clesrovimab is not recommended for this group.
This nirsevimab dose should be given shortly before the child’s second RSV season. This dose is recommended for:
- Children who were born prematurely and have chronic lung disease
- Children with severe immunocompromise
- Children with severe cystic fibrosis
- American Indian and Alaska Native children
Comparison of Immunizations
| Maternal Vaccine (Pfizer's Abrysvo) |
Infant RSV Antibody (nirsevimab or clesrovimab) |
|
|---|---|---|
| Who is it recommended for? | Women who are 32–36 weeks pregnant | Babies (ages <8 months) born to mothers who did not get a maternal RSV vaccine.
A small group of young children (ages 8–9 months) at increased risk of severe RSV (nirsevimab only).
|
| How does it work? | Mom passes protection (antibodies) to baby during pregnancy | Baby receives protection (antibodies) directly |
| When is it recommended in most of continental U.S.? | September – January | October – March |
| Who does it protect from severe RSV? | Baby | Baby |
| How long does protection last? | Approximately 6 months after birth | At least 5 months after immunization |
| Who should not get it? | Women with a history of severe allergic reaction to any component of the vaccine. | Children with a serious allergic reaction to any component of either infant RSV antibody OR Babies who are already protected because their mother received a maternal vaccine during pregnancy |
| Possible side effects | Common side effects: headache, nausea, pain at injection site, and more.
Possible risks under further study: hypertensive disorders of pregnancy, including pre-eclampsia (a dangerous high blood pressure condition).
|
Side effects are usually mild and end quickly (such as pain at the injection site).
Hypersensitivity reactions are uncommon but have been reported.
|
| Report adverse events to: | VAERS or call 1-800-822-7967 | FDA's Medwatch or call 1-800-FDA-1088 |
Timing of Immunization to Protect Infants
CDC recommends RSV immunizations during specific months to maximize protection during RSV season.
It is important for your baby to have protection before RSV season. RSV typically peaks between December and January.*
Download or print PDF: RSV Maternal/Pediatric Immunization Calendar
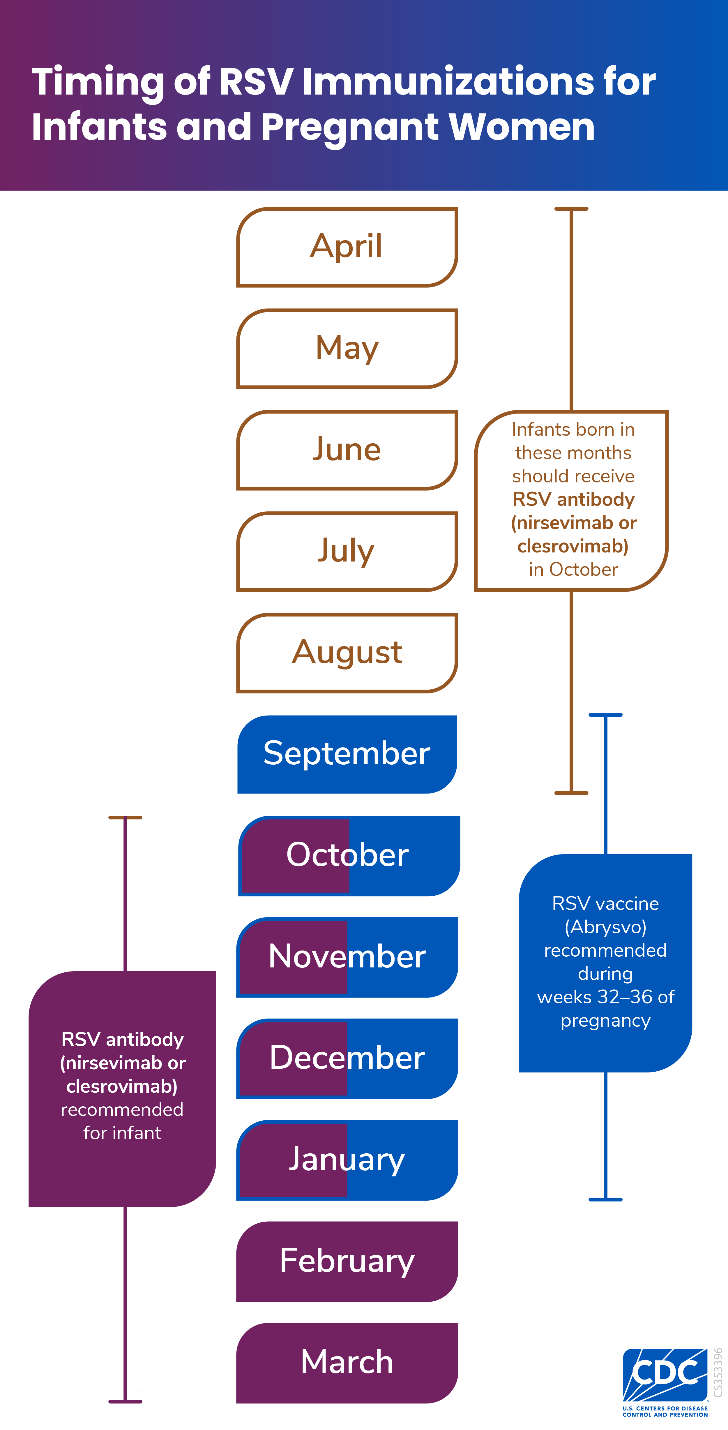
*Applies to most of the continental United States. Timing of Administration for RSV immunization may differ in certain areas. Learn more about seasonal administration of infant RSV antibodies here: RSV Immunization Guidance for Infants and Young Children.
How do I pay for RSV immunizations?
Vaccines for Children (VFC) program
Nirsevimab is covered by VFC, a federally funded program that provides vaccines at no cost to children who might not otherwise be vaccinated because of inability to pay. Children 18 years and under are eligible for the VFC Program if they belong to one or more of the following groups:
- Medicaid-eligible
- Uninsured
- Underinsured
- American Indian or Alaska Native
Private health insurance
Many private health insurance plans cover nirsevimab and most cover the maternal RSV vaccine, but there may be a cost to you depending on your plan. Contact your insurer to find out.
Medicaid
As of October 1, 2023, most people with coverage from Medicaid and the Children's Health Insurance Program (CHIP) will be guaranteed coverage of all vaccines recommended by the Advisory Committee on Immunization Practice at no cost to them.
