What to know
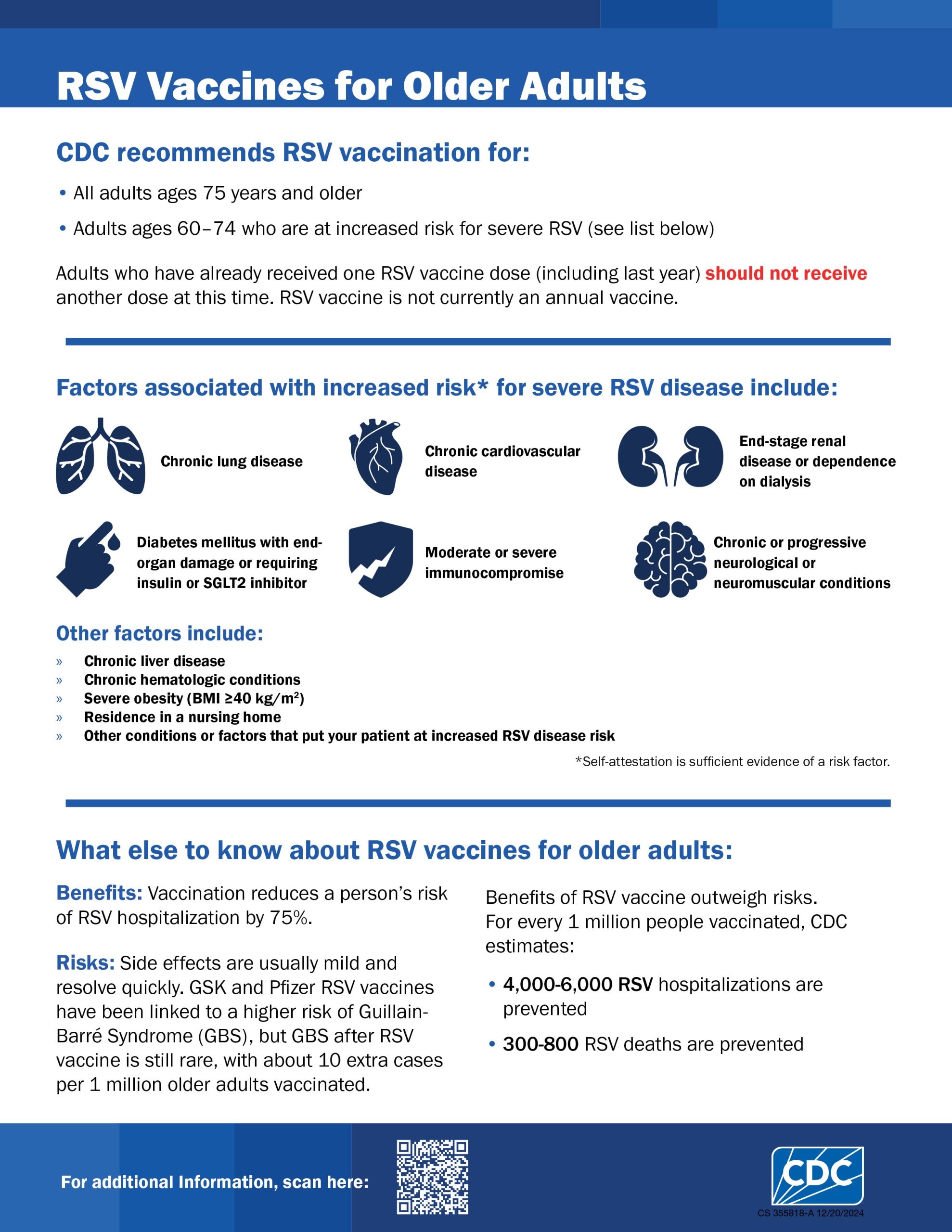
- CDC recommends a single dose of any FDA-licensed RSV vaccine for all adults ages 75 and older and adults ages 60–74 at increased risk of severe RSV.
- Three RSV vaccines are currently available for adults ages 60 and older: GSK’s Arexvy, Moderna’s mResvia, and Pfizer’s Abrysvo. Eligible older adults may receive any of the licensed RSV vaccines.
- Eligible adults can get an RSV vaccine at any time, but the best time to vaccinate patients is in late summer and early fall before RSV usually starts to spread in the community.
- The RSV vaccine is not currently an annual vaccine. People who have already received one dose (including last year) have completed their vaccination and should not receive another dose at this time.
Overview
CDC recommends a single dose of RSV vaccine for older adults to help prevent serious RSV infection and hospitalization.
RSV vaccination is recommended for the following adults:
- All adults ages 75 and older
- Adults ages 60–74 at increased risk of severe RSV disease
Conditions that increase risk of severe RSV
The following conditions increase the risk of severe RSV:*
- Chronic cardiovascular disease (e.g., heart failure, coronary artery disease, or congenital heart disease [excluding isolated hypertension])
- Chronic lung or respiratory disease (e.g., chronic obstructive pulmonary disease, emphysema, asthma, interstitial lung disease, or cystic fibrosis)
- End-stage renal disease or dependence on hemodialysis or other renal replacement therapy
- Diabetes mellitus complicated by chronic kidney disease, neuropathy, retinopathy, or other end-organ damage, or requiring treatment with insulin or sodium-glucose cotransporter-2 (SGLT2) inhibitor
- Neurologic or neuromuscular conditions causing impaired airway clearance or respiratory muscle weakness (e.g., poststroke dysphagia, amyotrophic lateral sclerosis, or muscular dystrophy [excluding history of stroke without impaired airway clearance])
- Chronic liver disease (e.g., cirrhosis)
- Chronic hematologic conditions (e.g., sickle cell disease or thalassemia)
- Severe obesity (body mass index ≥40 kg/m2)
- Moderate or severe immune compromise†
- Residence in a nursing home
- Other chronic medical conditions or risk factors that a healthcare provider determines would increase the risk for severe disease due to viral respiratory infection (e.g., frailty,§ situations in which healthcare providers have concern for presence of undiagnosed chronic medical conditions, or residence in a remote or rural community where transportation of patients with severe RSV disease for escalation of medical care is challenging¶)
* Patient attestation is sufficient evidence of the presence of a risk factor. Vaccinators should not deny RSV vaccination to a person because of lack of medical documentation.
† A list of moderately or severely immunocompromising conditions can be found in the COVID-19 vaccination interim clinical considerations. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html#immunocompromised
§ Frailty is a multidimensional geriatric syndrome that reflects a state of increased vulnerability to adverse health outcomes. Although no consensus definition exists, one frequently used tool for determination is the Fried frailty phenotype assessment, in which frailty is defined as a clinical syndrome with three or more of the following symptoms present: unintentional weight loss (10 lbs [4.5 kg] in the past year), self-reported exhaustion, weakness (grip strength), slow walking speed, or low physical activity.
¶ Healthcare providers caring for adults aged 60–74 years residing in these communities may use clinical judgement, knowledge of local RSV epidemiology, and community incidence of RSV-associated hospitalization to recommend vaccination for a broader population in this age group.
Available vaccines
There are currently three vaccines available:
CDC does not have a preferential recommendation for any specific vaccine. Eligible older adults should receive whichever vaccine is available.
Recommendations
Timing of RSV vaccination
Eligible adults who have not previously received RSV vaccination may be vaccinated at any time of year, but vaccination will have the most benefit if administered in late summer or early fall, just before the RSV season. In most of the continental United States, this corresponds to vaccination during August–October.
Number of doses
The RSV vaccine is not currently an annual vaccine, meaning eligible adults do not need to get a dose every RSV season. Currently, CDC recommends only a single dose of RSV vaccine for all adults ages 75 and older and for adults ages 60–74 with increased risk of severe RSV disease. Additional surveillance and evaluation activities are ongoing to determine whether adults might benefit from receiving additional RSV vaccine doses in the future.
The need for additional RSV vaccine doses will be evaluated by ACIP and CDC in the future; recommendations will be updated as needed.
Coadministration with other vaccines
RSV vaccines can be coadministered with other adult vaccines during the same visit.
Data on immunogenicity of RSV vaccines and other vaccines when coadministered are currently limited. These limited data show coadministration of RSV with other respiratory virus vaccines may result in lower antibody titers, but the clinical significance of this is unknown.
Coadministration of RSV vaccine with one or more adult vaccines during the same visit may increase common side effects (e.g., pain at the injection site, fever, headache, myalgia). CDC will continue to monitor vaccine safety and effectiveness to guide recommendations for coadministration.
When deciding whether to coadminister other vaccines with an RSV vaccine, providers should consider:
- whether the patient is recommended other vaccines
- the feasibility of the patient returning for vaccination during a separate visit
- risk for acquiring vaccine-preventable disease
- vaccine reactogenicity profiles
- patient preferences
Safety
Contraindications and precautions
GSK's Arexvy, Moderna’s mResvia, or Pfizer's Abrysvo should not be administered to a person with a history of severe allergic reaction, such as anaphylaxis, to any component of that vaccine. Healthcare providers should assess the potential risk of adverse events of each vaccine independently.
To learn more, see ACIP Contraindications Guidelines for Immunization, General Best Practice Guidelines for Immunization.
Reporting adverse events
Adverse events after RSV vaccination should be reported to the Vaccine Adverse Event Reporting System (VAERS), even if it is not clear that the vaccine caused the adverse event. Information on how to submit a report to VAERS is available at https://vaers.hhs.gov/index.html or by telephone at 1-800-822-7967.
To learn more about RSV vaccine safety, go to the following page:
Effectiveness
GSK’s Arexvy
In studies of the real-world effectiveness of Arexvy during the 2023–2024 RSV season, the first since licensure of Arexvy, Arexvy was approximately 77% effective in preventing RSV-associated emergency department encounters and 83% effective in preventing RSV-associated hospitalizations in adults 60 and older. In addition, effectiveness was demonstrated among adults ages 60 years or older with certain immunocompromising conditions and those with end-stage renal disease and in all adults 75 years or older.
Real-world effectiveness of Arexvy cannot yet be estimated during the second year following vaccination. However, clinical trial data showed durable protection against symptomatic RSV lower respiratory tract disease through approximately 23 months following vaccination, with some waning over time. CDC will continue to monitor real-world RSV vaccine effectiveness during each respiratory virus season.
Pfizer's Abrysvo
In studies of the real-world effectiveness of Abrysvo during the 2023–2024 RSV season, the first RSV season since licensure, Abrysvo was approximately 79% effective in preventing RSV-associated emergency department encounters and 73% effective in preventing RSV-associated hospitalizations in adults 60 and older. In addition, effectiveness was demonstrated among adults ages 60 years and older with certain immunocompromising conditions and those with end-stage renal disease and in all adults ages 75 years and older.
Real-world effectiveness of Abrysvo cannot yet be estimated during the second year following vaccination. However, clinical trial data showed durable protection against symptomatic RSV lower respiratory tract disease through approximately 18 months following vaccination, with some waning over time. CDC will continue to monitor real-world RSV vaccine effectiveness during each respiratory virus season.
Moderna's mResvia
Due to the recency of mResvia licensure, real-world vaccine effectiveness against RSV-associated hospitalization and other severe illness cannot yet be estimated. Vaccine efficacy data are available from one large phase 2/3 randomized, blinded placebo-controlled clinical trial in participants ages 60 and older. In this trial, efficacy of a single dose of mResvia against symptomatic RSV was approximately 80% during the first 4 months following vaccination and approximately 56% during the first 12 months after vaccination.
CDC will continue to monitor real-world RSV vaccine effectiveness during each respiratory virus season.
Adults younger than 60 years
On June 7, 2024, FDA approved use of GSK’s Arexvy in adults aged 50–59 years who are at increased risk for RSV-Lower Respiratory Tract Disease (LRTD). As of June 2024, ACIP judged that insufficient evidence was available to inform an RSV vaccine recommendation in adults aged 50–59 years who are at increased risk for RSV disease. Before deciding on a recommendation in adults younger than 60 years, ACIP expressed that the following items are needed: updated RSV vaccine safety analyses among adults 60 and older, including results from the full 2023–2024 RSV season in the FDA-CMS partnership analysis incorporating chart confirmation of GBS diagnoses; additional data on duration of protection from RSV vaccination and immune response after revaccination; and immunogenicity data in adults with immunocompromise. ACIP will review evidence and provide recommendations to CDC on RSV vaccination policy in this age group when additional data are available.
Resources
- ACIP Vaccine Recommendations
- RSV Vaccine Information Statement
- Safety Information for Respiratory Syncytial Virus (RSV) Vaccine
- CDC RSV Surveillance & Research
Download RSV HCP Older Adult Flyer (PDF)