
COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis
SYSTEMATIC REVIEW — Volume 19 — August 25, 2022
Ramya Nagarajan, MD1; Yuvaraj Krishnamoorthy, MD2; Sathish Rajaa, MD2; Vishnu Shankar Hariharan, MD3 (View author affiliations)
Suggested citation for this article: Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis 2022;19:210228. DOI: http://dx.doi.org/10.5888/pcd19.210228.
PEER REVIEWED
What is known on this topic?
Pre-existing comorbid conditions in COVID-19 patients are risk factors for developing severe disease and death.
What is added by this report?
Our literature review indicated that chronic liver disease (CLD) is associated with increased adverse clinical outcomes in terms of severity of disease and death among COVID-19 patients.
What are the implications for public health practice?
Results of our meta-analysis should encourage clinicians worldwide to provide extra attention and intensive care for patients with underlying CLD who develop COVID-19.
Abstract
Introduction
Pre-existing comorbid conditions in COVID-19 patients are risk factors for developing severe disease and death. We aimed to determine the association of chronic liver disease (CLD), a comorbid condition, with severity of disease and death among COVID-19 patients.
Methods
We searched for studies reporting COVID-19 outcomes among CLD and non-CLD patients in databases including Medline, EMBASE, ScienceDirect, Google Scholar, and Cochrane Library from inception of the pandemic until February 2022. Risk of bias assessment was conducted by using the Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies in meta-analyses. We conducted a meta-analysis with a random-effects model and reported pooled odds ratios (ORs) with 95% CIs.
Results
We included 40 studies with 908,032 participants. Most studies were conducted in China and the US. COVID-19 patients with CLD had significantly higher odds of having a severe form of COVID-19 (pooled OR = 2.44; 95% CI, 1.89–3.16) and death (pooled OR = 2.35; 95% CI, 1.85–3.00) when compared with COVID-19 patients without CLD.
Conclusion
The presence of CLD is significantly related to adverse clinical outcomes among COVID-19 patients in terms of severity and mortality. Clinicians should develop a comprehensive intervention plan to manage these high-risk patients and reduce COVID-19–related deaths.
Introduction
A coronavirus is a group of viruses that causes mild to severe respiratory tract infections in humans and animals (1). In recent times, we have witnessed outbreaks of severe acute respiratory syndrome (SARS) virus (2004), Middle East Respiratory Syndrome coronavirus (MERS-CoV) (2012), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (2019) that belong to this group of viruses (2). The SARS-CoV-2 outbreak is the most recent and was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 (3). As of May 16, 2021, 162 million cases and 3 million deaths were reported globally due to COVID-19 (4). The clinical features range from asymptomatic infection to severe pneumonia and death. However, patients who have comorbidities are more likely to have a severe form of the condition or to die (5).
Chronic liver disease (CLD) is marked by the gradual destruction of liver parenchyma over time. Various factors cause it; the most common are alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), chronic viral hepatitis, and genetic and autoimmune causes (6). Understanding the conditions that lead to severe disease and death among COVID-19–infected people is critical with the evolving pandemic (7). COVID-19 infection highlights the pre-existing weaknesses of the individual organ systems (8), making it logical to postulate that people with CLD may be susceptible to more severe respiratory infections or be at increased risk of death. In addition, it has been proposed that metabolic-associated fatty liver disease (MAFLD) or NAFLD is associated with significant or advanced fibrosis that might exacerbate the “cytokine storm” induced by the COVID-19 infection (9). The mechanism behind this is probably through the release of various proinflammatory hepatokines, which might contribute mechanistically to developing a severe form of COVID-19 infection (9). Several studies have found that hospitalized COVID-19 patients with CLD had an acute rise in liver enzymes, which results in a severe condition requiring mechanical ventilation and even leading to death (10–12). Existing evidence on COVID-19 outcomes among CLD patients has reported mixed results, making it difficult to determine a prognosis for these patients (10–13). Hence, we conducted a systematic review and meta-analysis to find the association between CLD and the severity of and mortality caused by COVID-19.
Methods
This was a systematic review and meta-analysis of observational studies and was performed according to the Preferred Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (14). The study protocol was registered in the PROSPERO database (registration ID: CRD42021291761).
Eligibility criteria
We included studies with any of the following study designs: prospective or retrospective cohort, case control, and cross-sectional. Only published full-text studies were included; conference abstracts, unpublished data, and gray literature were excluded. Studies conducted among COVID-19 patients were included; studies among COVID-19 patients with comorbidities other than CLD were excluded.
Studies reporting the COVID-19 outcomes among CLD and non-CLD patients were included. CLD patients are diagnosed with the condition by clinical examination, laboratory or radiologic examination, or all 3 investigations. The CLD conditions most commonly found in COVID-19 patients included in our review were cirrhosis, viral hepatitis, NAFLD, and MAFLD. Studies reporting the diagnosis of CLD based on previous medical records were also included in the review.
Outcomes were the 1) severity of COVID-19 and 2) mortality due to COVID-19. The severity of the COVID-19 condition can be graded based on any of the following patient criteria: respiratory rate >30 breaths/min; oxygen saturation (SpO2) <93%; oxygenation index (PaO2/FiO2) ≤300 mm Hg; intensive care unit stay required; or mechanical ventilation (15). Studies reporting any of the outcomes mentioned above were included in our review.
Search strategy
We conducted a comprehensive, systematic, and extensive search in the electronic databases Medline, EMBASE, ScienceDirect, Google Scholar, and Cochrane Library. We selected the terms required for the search during the protocol stage. We used both the medical subject headings (MeSH) and free-text words while searching these databases. The keywords and their synonyms were searched using appropriate truncations, wildcards, and proximity searching. The terms used to search were “liver disease”/exp OR “hepatic disease”:ti,ab OR “hepatic disorder”:ti,ab OR “hepatopathy”:ti,ab OR “liver cell disease”:ti,ab OR “liver disease”:ti,ab OR “liver diseases”:ti,ab OR “liver disorder”:ti,ab OR “liver illness”:ti,ab) AND “coronavirus disease 2019”/exp AND (“mortality”/exp OR “excess mortality” OR “mortality” OR “mortality model” OR “disease severity”/exp OR “disease severity” OR “illness severity” OR “severity, illness” OR “cause of death”/exp OR “cause of death” OR “cause, death” OR “death cause” OR “death caused” OR “mortality cause” OR “death”/exp OR “death” OR “mortality”. We also searched for crucial concepts using corresponding subject headings in each database. The last search was carried out by combining the individual search results using appropriate Boolean operators (“OR” and “AND”). The search was narrowed down using the available filters on the type of studies. We restricted the search from the inception of the pandemic to February 2022 and published in English only (Supplementary Table 1 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). Bibliographies of the retrieved articles were also hand-searched to identify any themes missed during the database search.
Study selection process
This process involved 3 stages:
- Primary screening: Two independent investigators (R.N. and Y.K.) performed preliminary screening of title, abstract, and keywords by executing the literature search. Full-text articles were retrieved for the studies shortlisted on the basis of the eligibility criteria.
- Secondary screening: The same 2 investigators (R.N. and Y.K.) screened the full text of these retrieved studies and assessed them against the review’s eligibility criteria. Studies that satisfied all the eligibility criteria concerning design, participants, exposure, and outcome were included.
- Finalizing the study selection: Disagreements during the screening process between the investigators were resolved. A final consensus on the inclusion of studies was reached with the help of another investigator (S.R.).
Data extraction
Data were extracted manually from the included studies using a structured data extraction form that was developed and pilot tested during the protocol stage. We extracted the following data: general information, such as author and year of publication; information related to methods, such as study design, setting, sample size, sampling strategy, study participants, inclusion and exclusion criteria, outcome assessment method, and quality-related information; and information related to outcomes, such as patients’ severity of disease and mortality rates. Data were entered by the investigator (S.R.), and the entry was double checked by the secondary investigator (V.H.).
Risk of bias assessment
Two independent investigators (S.R. and V.H.) used the Newcastle-Ottawa Scale to assess the risk of bias and quality of nonrandomized studies in meta-analyses under 3 domains: selection, comparability, and outcome (16). The quality of the study was graded as good, fair, or poor based on the scores obtained under each domain.
Data synthesis
We used Stata version 16 (StataCorp LLC) to conduct the meta-analysis. Because all outcomes were dichotomous, the number of events and participants in each group were entered to obtain the pooled effect estimate in terms of odds ratios (ORs) with 95% CIs and prediction intervals (PIs). We used the random-effects model with the restricted maximum likelihood method to calculate the weights of individual studies (17) because of the clinical and methodologic heterogeneity among the included studies. We used the command meta esize to compute the summary statistic; it automatically adjusts for zero cells by adding 0.5 to all cells in a 2-by-2 table that contains a zero value while computing the summary statistic. Evidence of between-study variance due to heterogeneity was assessed through the ꭓ2 test of heterogeneity and I2 statistics to quantify the inconsistency. I2 less than 25% is mild, 25% to 75% is moderate, and more than 75% is considered substantial heterogeneity (17). Study-specific and pooled estimates were graphically represented through a forest plot. We also performed a sensitivity analysis to assess the robustness of the results by removing the studies one at a time and checking for any significant variation in the results. We also performed subgroup analysis on the basis of each type of CLD.
We conducted univariable meta-regression with the study-level characteristics using the metareg package in Stata. Publication bias was assessed for each outcome using the funnel plot and Doi plot for visual interpretation and Egger test and Luis Furuya-Kanamori asymmetry index (LFK index) for statistical interpretation (18). Asymmetry of the funnel plot and Doi plot and P value less than .10 in the Egger test indicates the possibility of publication bias. On the basis of the LFK index value, the possibility of publication bias was classified as no asymmetry (value within ±1), minor asymmetry (value out of ±1 but within ±2), and major asymmetry (value more than ±2) (18).
Results
We found 3,659 records through the systematic literature search and deemed 221 of those studies relevant for full-text retrieval. We also retrieved the full text for 36 articles obtained through manual searching of the bibliographies in the retrieved studies. During the second screening stage, 40 studies with 908,032 participants met the eligibility criteria and were included in the analysis (Figure 1) (8–12,15,19–52). This study was reported as per the PRISMA statement guidelines (Supplementary Table 2 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
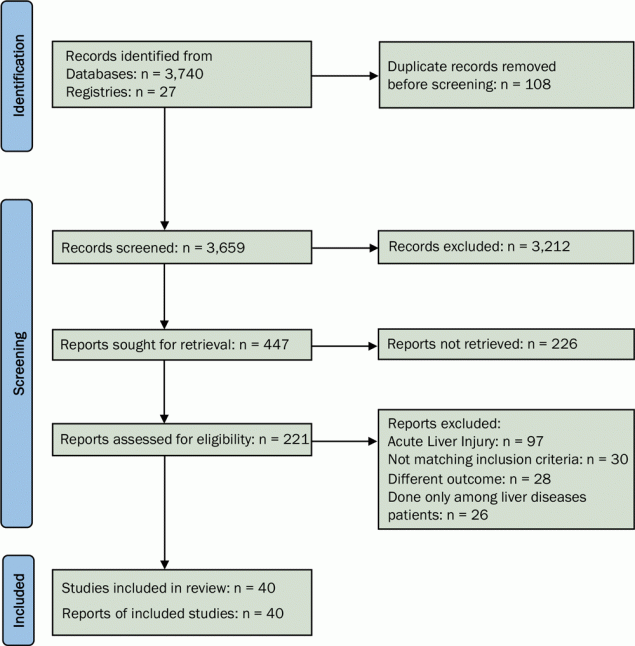
![]()
Figure 1.
PRISMA flowchart showing the identification of studies for analysis of the association of chronic liver disease with severity of disease and mortality among COVID-19 patients. Abbreviation: PRISMA, Preferred Items for Systematic Reviews and Meta-Analyses. [A text description of this figure is available.]
In total, 909,831 participants were found in the included studies, with a sample size ranging from 41 to 259,110 (Table). Among the 40 studies included, 15 reported on mortality due to COVID-19, 14 reported on the severity of COVID-19, and 11 reported both on severity and mortality. All included studies were retrospective; most studies were conducted in China (n = 14) and the US (n = 10). Half (21 of 40) of the included studies were low-quality (ie, per the Newcastle-Ottawa Scale) (Supplementary Table 3 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing) (16).
Association between CLD and COVID-19 outcomes
Severity
In our analysis, 25 studies reported the severity of the CLD and the non-CLD groups (9,10,19–21,23,24,27–29,31,32,34,36,37,40–44,46,49–52). The pooled OR was 2.44 (95% CI, 1.89–3.16; I2 = 91.3%; 95% PI, 0.79–7.55) (Figure 2), indicating that the odds of developing severe disease among COVID-19 patients with CLD were 2.44 times higher than among those without CLD. High heterogeneity was found between the studies reporting the severity outcome (I2 = 91.3%, P < .001).
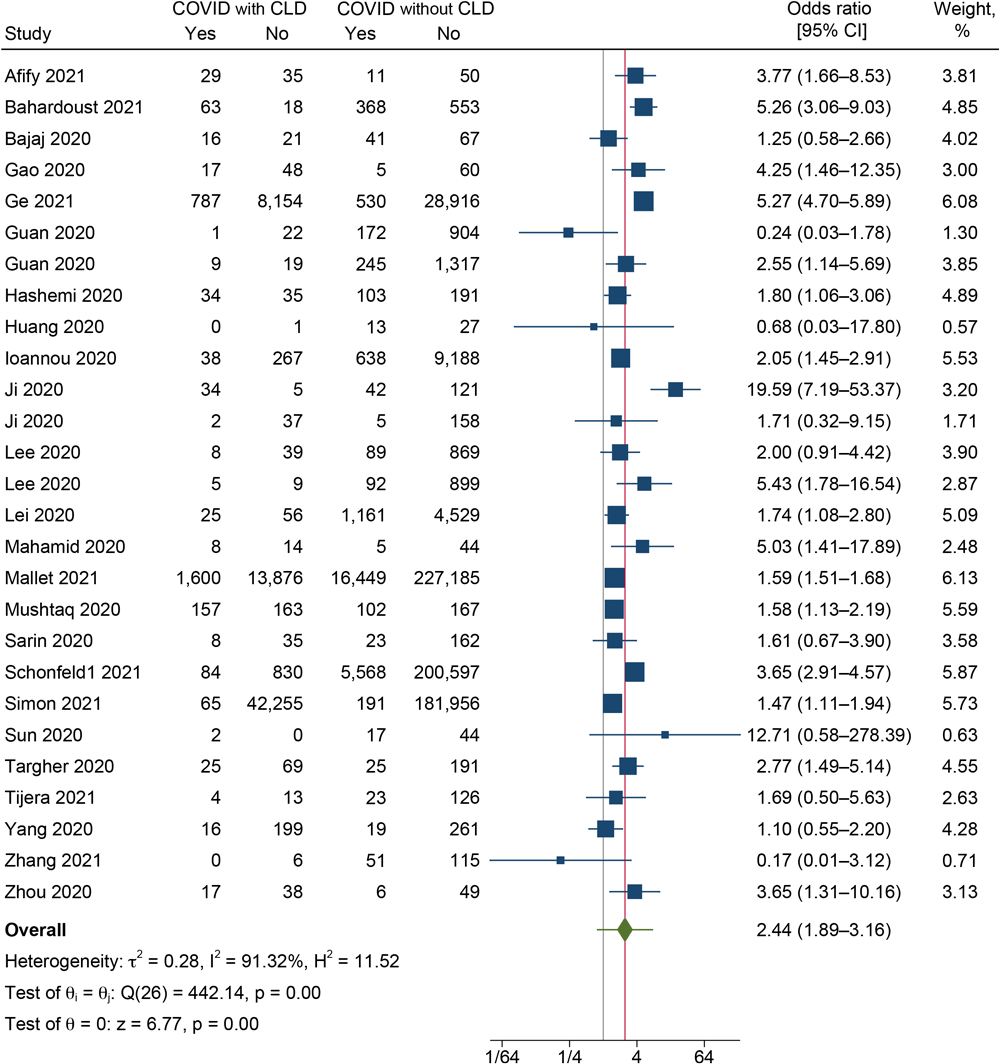
![]()
Figure 2.
Forest plot showing the difference in severity between COVID-19 patients with and without CLD (N = 27). Abbreviation: CLD, chronic liver disease. [A text description of this figure is available.]
Subgroup analysis showed that COVID-19 patients with NAFLD had the highest odds of COVID-19 severity (pooled OR = 5.60; 95% CI, 1.52–20.64), followed by MAFLD (pooled OR = 3.20; 95% CI, 1.99–5.14) and cirrhosis (pooled OR = 3.09; 95% CI, 1.95–4.89) (Supplementary Figure 1 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). COVID-19 patients who had viral hepatitis did not have significantly higher odds of having a severe form of COVID-19 (pooled OR = 1.29; 95% CI, 0.36–4.63). Subgroup analysis by study design showed significantly higher odds of severity in the studies following cohort design (pooled OR = 3.10; 95% CI, 2.08–6.60; P = .001) (Supplementary Figure 2 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Results of the univariable meta-regression showed that geographic region, type of CLD, quality of study, year of publication, sample size, and mean age of participants were not significantly associated with the pooled effect size and cannot explain the substantial heterogeneity in the results (Supplementary Table 4 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Publication bias was graphically checked by funnel plot and Doi plot (Supplementary Figures 3 and 4 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). The funnel plot showed no sign of asymmetry, and it was also statistically proved by Egger test (P = .36); the Doi plot also showed no asymmetry, with an LFK index of 0.93. Sensitivity analysis showed no significant variation in the magnitude or direction of the outcome, indicating a lack of influence of a single study on the overall pooled estimate (Supplementary Figure 5 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Mortality
In total, 26 studies reported on the mortality outcome among CLD and non-CLD patients (8,10–12,15,19,20,22,24–26,29,30,33,35,38,39,41,44–51). The pooled OR was 2.35 (95% CI, 1.84–3.00; I2 = 96.26%; 95% PI, 0.76–7.18) (Figure 3), indicating that COVID-19 patients with CLD had 2.35 times higher odds of dying as patients without CLD. We found substantial heterogeneity between the studies reporting the mortality outcome (I2 = 96.3%, P < .001).
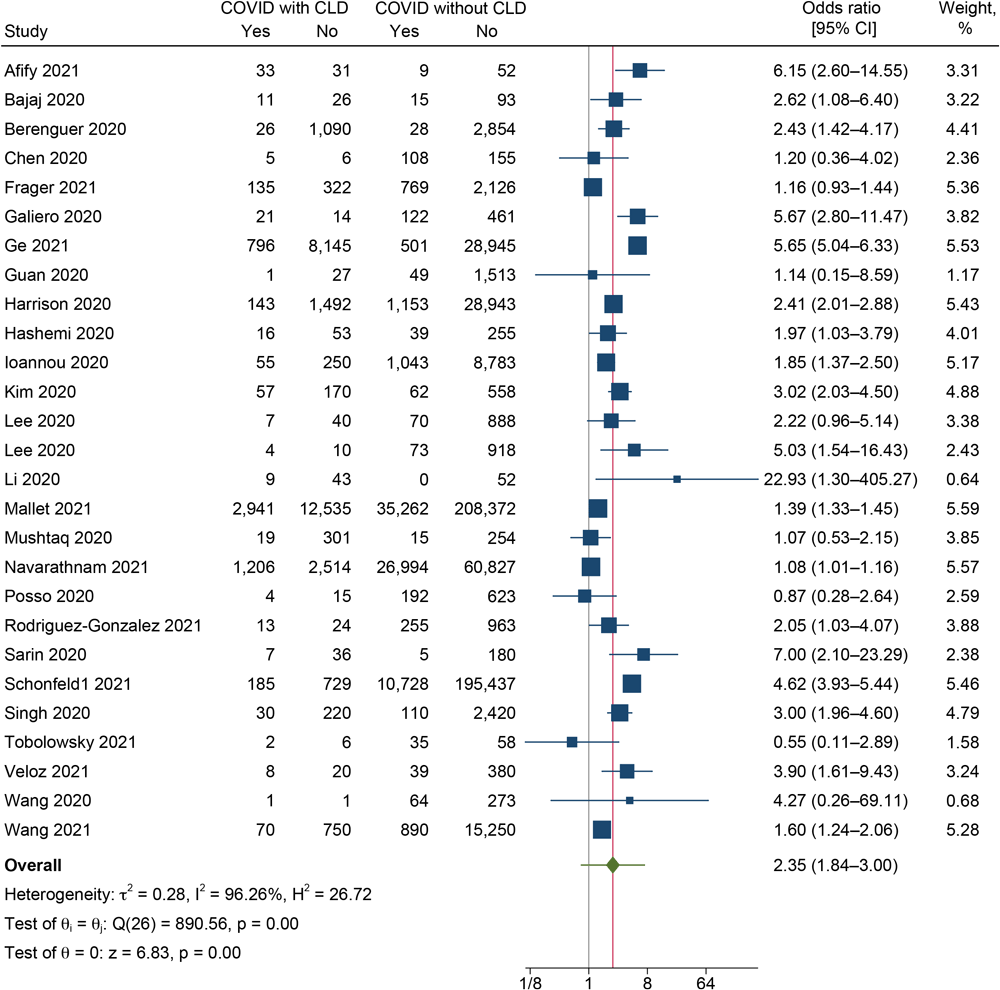
![]()
Figure 3.
Forest plot showing the difference in mortality between COVID-19 patients with and without chronic liver disease (CLD) (N = 27). [A text description of this figure is available.]
Subgroup analysis based on the type of CLD could not be done because only cirrhosis had enough studies to give a pooled estimate (all the other studies reporting mortality outcomes were conducted among CLD patients without categorizing them based on the type of CLD). We found that COVID-19 patients with cirrhosis had 3.51 times higher odds of dying as patients without cirrhosis (pooled OR = 3.51; 95% CI, 2.41–5.10) (Supplementary Figure 6 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). Subgroup analysis by study design showed significantly higher odds of severity among the studies conducted using a cohort (pooled OR = 2.94; 95% CI, 2.09–4.13; P < .001) and a retrospective cohort design (pooled OR = 2.19; 95% CI, 1.51–3.17; P < .001) (Supplementary Figure 7 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Univariable meta-regression showed that only the mean age of the patients had a significant association with the pooled effect size (P = .01) and explained 48.3% of the between-study variability (Supplementary Figure 8 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). None of the other factors were significantly associated with the pooled effect size and cannot explain the substantial heterogeneity in the results (Supplementary Table 5 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Publication bias was graphically checked by funnel plot and Doi plot (Supplementary Figures 9 and 10 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing). The funnel plot showed signs of asymmetry, with the Egger test (P = .10) also showing signs of possible publication bias. The Doi plot showed significant asymmetry, with an LFK index of 4.47. Sensitivity analysis showed no significant variation in the magnitude or direction of the outcome, indicating a lack of influence of a single study on the overall pooled estimate (Supplementary Figure 11 available at: https://drive.google.com/drive/folders/1mVlexUbFzmHcfvi44LTFi18OmTnMZXtT?usp=sharing).
Discussion
We found that the risk of COVID-19 severity and death was twice as high among CLD patients than among non-CLD patients. Similar results were observed in a review conducted by Wu and Yang in which COVID-19 patients with CLD had more than 4 times the chance of developing severe disease and almost twice the chance of dying compared with non-CLD COVID-19 patients (53). Reviews conducted by Sharma et al and Yadav et al also found higher chances of developing severe disease and death among COVID-19 hospitalized patients with pre-existing liver diseases. Patients with elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were also reported to have higher chances of severe illness and death (54,55). However, a review conducted by Lippi et al states otherwise; no significant changes between liver disease and non–liver disease groups were found with respect to COVID-19 outcomes. However, the studies included in that analysis were limited, so its results should be interpreted cautiously (56).
Subgroup analysis based on the type of CLD showed that patients with NAFLD had the highest risk of severe disease, followed by those with MAFLD and cirrhosis. The estimates were also similar compared with the previous review findings (57–59). A similar analysis could not be done for mortality outcomes because of limitations in the number of studies. Still, research based on cirrhosis showed a higher effect size than the overall pooled estimate. Understanding the mechanism behind this finding is essential because it will help explain the reason for the association obtained in all the existing evidence. Subgroup analysis was also performed based on the study design adapted to conduct the study. We found higher odds of severity and mortality among studies adapting a cohort design. Though the estimates obtained from a cohort design are considered to be more powerful compared with a case-control or cross-sectional design, we are unclear about how the study design influences the severity and mortality outcome in our review (60). We recommend conducting further studies to evaluate the influence of study design in the outcome of severity and mortality studies.
The possible reason for the higher risk of severity among NAFLD patients could be the complex interplay of chronic active inflammatory pathways between the COVID-19–associated cytokine storm and NAFLD (59). Injury caused by the accumulation of fat in the liver could exacerbate the cytokine storm and worsen the prognosis of patients (61). In addition, liver fibrosis has been linked with a higher risk of severity among COVID-19 patients (62). Hence, liver fat accumulation and subsequent fibrosis may be the reasons for NAFLD patients’ more deficient outcomes. A similar mechanism was also observed for MAFLD because it was found to exacerbate the virus-induced inflammatory cytokine storm by increased reactive oxygen production and hepatic release of the proinflammatory cytokines in the COVID-19 patients (57,63). Finally, the possible pathogenesis behind the cirrhosis patients having a higher rate of severity and death following COVID-19 infection could be the excess systemic inflammation, intestinal dysbiosis, cirrhosis-induced immune dysfunction, and coagulopathies (59). Despite all these reasonings and mechanisms, determining the reason for such differential risk associated with different CLD patients is necessary. This determination can be achieved by performing proper longitudinal research in such patients and developing a deeper understanding of this issue.
The major strength of our review was the rigorous literature search and methodology followed to provide reliable estimates. In addition, this review adds to the limited evidence available on the prognostic importance of CLD among COVID-19 patients. We also performed additional subgroup analyses to stratify the risk of adverse outcomes based on the type of CLD and study design, meta-regression to explore the source of heterogeneity, and sensitivity analysis to check the robustness of our results.
Our study had limitations and findings should be interpreted cautiously, considering the difference in methods and quality across the included studies. Although the review by Mauvais-Jarvis et al stated the influence of gender over disease profile globally and the importance of having gender representation in medical research, our search found that data relevant to evaluating the severity of disease and mortality caused by COVID-19 in CLD patients by gender was lacking in the included studies, which is a limitation in our review (64). Our analysis also found significant between-study variability (significant χ2 test for heterogeneity and I2 statistics) for both outcomes. Such high heterogeneity can be attributed to the methodologic differences between the included studies, such as analysis by type of CLD, setting, sample size, and mean age. Meta-regression analysis did not indicate a significant source of heterogeneity for severity outcome and found only mean age as an essential source of heterogeneity for mortality outcome. In addition, we found substantial publication bias for the mortality outcome and found that most of the studies included in our review were of lower quality, which might further limit the generalizability of our study findings.
Although our results provide crucial information for better understanding the association of CLD and adverse COVID-19 outcomes, a need exists to perform longitudinal studies to establish the temporality of association and causal link between CLD and adverse clinical effects in the COVID-19 patients. Understanding this link will break a crucial barrier in managing COVID-19 patients and help prevent many deaths worldwide.
Our findings have implications for clinical management. Although patients with any liver pathology have some adverse outcomes, the magnitude almost doubles if the patients have CLD. Results of our meta-analysis should encourage clinicians worldwide to provide extra attention and intensive care for patients with CLD, who should be provided with advanced management to prevent adverse clinical outcomes.
Acknowledgments
The authors have no conflicts of interest to declare. No funding was secured for this study, and no copyrighted materials or tools were used in this research.
Author Information
Corresponding Author: Ramya Nagarajan, MD, Indian Council of Medical Research, National Institute of Epidemiology, Chennai, India. Telephone: 91-748-340-2558. Email: nagarajanramya55@gmail.com.
Author Affiliations: 1Indian Council of Medical Research, National Institute of Epidemiology, Chennai, India. 2Department of Community Medicine, ESIC Medical College and PGIMSR, K.K. Nagar, Chennai, Tamil Nadu, India. 3Department of General Medicine, Hindu Mission Hospital, Chennai, Tamil Nadu, India.
References
- Fenner and White’s Medical Virology: coronavirus. Academic Press; 2017. Accessed May 10, 2022. https://www.sciencedirect.com/topics/veterinary-science-and-veterinary-medicine/coronavirus
- Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: emergence, transmission, and characteristics of human coronaviruses. J Adv Res 2020;24:91–8. CrossRef PubMed
- World Health Organization. Coronavirus disease 2019 situation report 51. World Health Organization; 2020. Accessed May 10, 2022. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn =1ba62e57_10
- World Health Organization. WHO Coronavirus (COVID-19) dashboard. World Health Organization; 2021. Accessed May 10, 2022. https://covid19.who.int/
- Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, et al. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 2021;76(2):428–55. CrossRef PubMed
- Sharma A, Nagalli S. Chronic liver disease. StatPearls 2021 Nov 25. http://www.ncbi.nlm.nih.gov/pubmed/32119484
- Centers for Disease Control and Prevention. What to know about liver disease and COVID-19. Accessed May 10, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/liver-disease.html
- Berenguer J, Ryan P, Rodríguez-Baño J, Jarrín I, Carratalà J, Pachón J, et al. ; COVID-19@Spain Study Group. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect 2020;26(11):1525–36. CrossRef PubMed
- Bahardoust M, Heiat M, Khodabandeh M, Karbasi A, Bagheri-Hosseinabadi Z, Ataee MH, et al. Predictors for the severe coronavirus disease 2019 (COVID-19) infection in patients with underlying liver disease: a retrospective analytical study in Iran. Sci Rep 2021;11(1):3066. CrossRef PubMed
- Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut 2021;70(3):531–6. CrossRef PubMed
- Frager SZ, Szymanski J, Schwartz JM, Massoumi HS, Kinkhabwala M, Wolkoff AW. Hepatic predictors of mortality in severe acute respiratory syndrome coronavirus 2: role of initial aspartate aminotransferase/alanine aminotransferase and preexisting cirrhosis. Hepatol Commun 2021;5(3):424–33. CrossRef PubMed
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. CrossRef PubMed
- Klein F. Risikofaktor komorbiditäten bei COVID-19 — erkrankung. Pneumologie 2020;74(10):640. German.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. CrossRef PubMed
- Galiero R, Pafundi PC, Simeon V, Rinaldi L, Perrella A, Vetrano E, et al. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: findings from COVOCA study. Di Gennaro F, editor. PLoS One 2020 Dec 10;15(12):e0243700.
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. University of Ottowa. Accessed May 10, 2022. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions version 63. Cochrane; 2022. Accessed May 10, 2022. https://www.training.cochrane.org/handbook
- Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid-Based Healthc 2018;16(4):195–203. CrossRef PubMed
- Ioannou GN, Liang PS, Locke E, Green P, Berry K, O’Hare AM, et al. Cirrhosis and severe acute respiratory syndrome coronavirus 2 infection in US veterans: risk of infection, hospitalization, ventilation, and mortality. Hepatology 2021;74(1):322–35. CrossRef PubMed
- Lee YR, Kang MK, Song JE, Kim HJ, Kweon YO, Tak WY, et al. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: a multicenter study in South Korea. Clin Mol Hepatol 2020;26(4):562–76. CrossRef PubMed
- de la Tijera HF, Servín-Caamaño A, Reyes-Herrera D, Flores-López A, Robiou-Vivero EJA, Martínez-Rivera F, et al. Impact of liver enzymes on SARS-CoV-2 infection and the severity of clinical course of COVID-19. Liver Res 2021;5(1):21–7. CrossRef PubMed
- Veloz G, Cordero Ruiz P, Ríos-Villegas MJ, Del Pino Bellido P, Bravo-Ferrer J, Galvés Cordero R, et al. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig 2021;113(2):103–9. PubMed
- Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol 2020;73(2):451–3. CrossRef PubMed
- Mallet V, Beeker N, Bouam S, Sogni P, Pol S, Mallet V, et al. ; Demosthenes Research Group. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol 2021;75(4):848–55. CrossRef PubMed
- Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80(6):639–45. CrossRef PubMed
- Li C, Chen Q, Wang J, Lin H, Lin Y, Lin J, et al. Clinical characteristics of chronic liver disease with coronavirus disease 2019 (COVID-19): a cohort study in Wuhan, China. Aging (Albany NY) 2020;12(16):15938–45. CrossRef PubMed
- Simon TG, Hagström H, Sharma R, Söderling J, Roelstraete B, Larsson E, et al. Risk of severe COVID-19 and mortality in patients with established chronic liver disease: a nationwide matched cohort study. BMC Gastroenterol 2021;21(1):439. CrossRef PubMed
- Zhou YJ, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int 2020;40(9):2160–3. CrossRef PubMed
- Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. ; China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55(5):2000547. CrossRef PubMed
- Rodriguez-Gonzalez CG, Chamorro-de-Vega E, Valerio M, Amor-Garcia MA, Tejerina F, Sancho-Gonzalez M, et al. COVID-19 in hospitalised patients in Spain: a cohort study in Madrid. Int J Antimicrob Agents 2021;57(2):106249. CrossRef PubMed
- Sun Y, Dong Y, Wang L, Xie H, Li B, Chang C, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun 2020;112:102473. CrossRef PubMed
- Yang R, Gui X, Ke H, Gao S, Luo M, Xiong Y. The indicative role of markers for liver injury on the severity and prognosis of coronavirus disease 2019 patients. Eur J Gastroenterol Hepatol 2021;33(Suppl 1):e176–82. CrossRef PubMed
- Tobolowsky FA, Bardossy AC, Currie DW, Schwartz NG, Zacks RLT, Chow EJ, et al. Signs, symptoms, and comorbidities associated with onset and prognosis of COVID-19 in a nursing home. J Am Med Dir Assoc 2021;22(3):498–503. CrossRef PubMed
- Zhang Q, Wang Z, Lv Y, Zhao J, Dang Q, Xu D, et al. Clinical features and prognostic factors of patients with COVID-19 in Henan Province, China. Hum Cell 2021;34(2):419–35. CrossRef PubMed
- Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med 2020;17(9):e1003321. CrossRef PubMed
- Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol 2021;36(1):204–7. CrossRef PubMed
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506. CrossRef PubMed
- Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center study. Clin Gastroenterol Hepatol 2021;19(7):1469–1479.e19. CrossRef PubMed
- Navaratnam AV, Gray WK, Day J, Wendon J, Briggs TWR. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med 2021;9(4):397–406. CrossRef PubMed
- Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020;72(2):389–98. CrossRef PubMed
- Sarin SK, Choudhury A, Lau GK, Zheng M-H, Ji D, Abd-Elsalam S, et al. ; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int 2020;14(5):690–700. CrossRef PubMed
- Targher G, Mantovani A, Byrne CD, Wang X-B, Yan H-D, Sun Q-F, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020;69(8):1545–7. CrossRef PubMed
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–20. CrossRef PubMed
- Mushtaq K, Khan MU, Iqbal F, Alsoub DH, Chaudhry HS, Ata F, et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression — the debate continues. J Hepatol 2021;74(2):482–4. CrossRef PubMed
- Posso M, Comas M, Román M, Domingo L, Louro J, González C, et al. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a university hospital in Spain. Arch Bronconeumol 2020;56(11):756–8.
- Schönfeld D, Arias S, Bossio JC, Fernández H, Gozal D, Pérez-Chada D. Clinical presentation and outcomes of the first patients with COVID-19 in Argentina: results of 207,079 cases from a national database. PLoS One 2021;16(2):1–16.
- Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020;159(2):768–771.e3. CrossRef PubMed
- Wang Q, Davis PB, Xu R. COVID-19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine 2021;31:100688. CrossRef PubMed
- Afify S, Eysa B, Hamid FA, Abo-Elazm OM, Edris MA, Maher R, et al. Survival and outcomes for co-infection of chronic hepatitis C with and without cirrhosis and COVID-19: a multicenter retrospective study. World J Gastroenterol 2021;27(42):7362–75. CrossRef PubMed
- Ge J, Pletcher MJ, Lai JC, Harper JR, Chute CG, Haendel MA; N3C Consortium. Outcomes of SARS-CoV-2 Infection in patients with chronic liver disease and cirrhosis: a national COVID cohort collaborative study. Gastroenterology 2021;161(5):1487–1501.e5. CrossRef PubMed
- Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int 2020;40(10):2515–21. CrossRef PubMed
- Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, et al. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol 2021;33(12):1578–81. CrossRef PubMed
- Wu Z-H, Yang D. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res 2020;25(54). CrossRef PubMed
- Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, et al. Liver disease and outcomes among COVID-19 hospitalized patients — a systematic review and meta-analysis. Ann Hepatol 2021;21:100273. CrossRef PubMed
- Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut 2021;70(4):807–9. CrossRef PubMed
- Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in coronavirus disease 2019 (COVID-19): a pooled analysis. Eur J Gastroenterol Hepatol 2021;33(1):114–5. CrossRef PubMed
- Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, et al. Metabolic associated fatty liver disease is associated with an increased risk of severe COVID-19: a systematic review with meta-analysis. Front Med (Lausanne) 2021;8:626425. CrossRef PubMed
- Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: a meta-analysis. Dig Liver Dis 2021;53(2):153–7. CrossRef PubMed
- Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: a pooled analysis. SN Compr Clin Med 2020;2(12):2726–9. CrossRef PubMed
- Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg 2010;126(6):2234–42. CrossRef PubMed
- Roca-Fernandez A, Dennis A, Nicolls R, McGonigle J, Kelly M, Banerjee R. High liver fat associates with higher risk of developing symptomatic COVID-19 infection — initial UK biobank observations. BMJ 2020;65(2):229–33. CrossRef
- Targher G, Mantovani A, Byrne CD, Wang X-BB, Yan H-DD, Sun Q-FF, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020;69(8):1545–7. CrossRef PubMed
- Assante G, Williams R, Youngson NA. Is the increased risk for MAFLD patients to develop severe COVID-19 linked to perturbation of the gut-liver axis? J Hepatol 2021;74(2):487–8. CrossRef PubMed
- Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020;396(10250):565–82. CrossRef PubMed
Table
| Reference no. | Study | Country | Design | Mean age, y | Sample size | CLD criteria | COVID-19 severity criteria | Outcome assessed | Study quality |
|---|---|---|---|---|---|---|---|---|---|
| 49 | Afify et al (2021) | Egypt | RCS | NA | 125 | NA | ICU admission | Severity and mortality | Poor |
| 9 | Bahardoust et al (2021) | Iran | Case-control study | 60 | 1,002 | Previous medical records | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity | Good |
| 10 | Bajaj et al (2020) | US | Matched cohort study | 61 | 145 | Prior liver biopsy, evidence of frank hepatic decompensation, radiologic evidence of a nodular liver and/or features of portal hypertension or endoscopic evidence of varices | ICU transfer | Severity and mortality | Good |
| 8 | Berenguer et al (2020) | Spain | RCS | 70 | 3,998 | Previous medical records | NA | Mortality | Poor |
| 12 | Chen et al (2020) | China | RCS | 62 | 274 | Previous medical records | NA | Mortality | Poor |
| 11 | Frager et al (2021) | US | RCS | 64.8 | 3,352 | FIB-4 of >3.25 and/or Fibro Scan transient elastography results of >12.5 kPa | NA | Mortality | Poor |
| 15 | Galiero et al (2020) | Italy | RCS | 65 | 618 | Previous records and laboratory examination | NA | Mortality | Good |
| 36 | Gao et al (2020) | China | Cohort study | 46 | 130 | Presence of steatosis by histology or imaging | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg/mech ventilation/shock/ICU | Severity | Good |
| 50 | Ge et al (2021) | US | Cohort study | NA | 38,387 | Documentation of at least 1 OMOP concept identifier corresponding to previously validated ICD-10-CM codes for liver diseases at any time before the index date | NA | Severity and mortality | Good |
| 43 | Guan et al (2020) | China | RCS | 47 | 1,099 | Previous medical records | American Thoracic Society guidelines for community-acquired pneumonia | Severity | Poor |
| 29 | Guan et al (2020) | China | RCS | 48.9 | 1,590 | Previous medical records | American Thoracic Society guidelines for community-acquired pneumonia | Severity and mortality | Poor |
| 35 | Harrison et al (2020) | US | RCS | 50 | 31,731 | Previous medical records | NA | Mortality | Poor |
| 51 | Hashemi et al (2020) | US | RCS | 63.4 | 363 | Manual review of laboratory, imaging and/or histopathological data | ICU admission | Severity and mortality | Good |
| 37 | Huang et al (2020) | China | Cohort study | 49 | 41 | Laboratory investigation (LFT) | ICU admission | Severity | Poor |
| 19 | Ioannou et al (2020) | US | Cohort study | NA | 10,131 | Previous medical records | Need for mechanical ventilation | Severity and mortality | Good |
| 23 | Ji et al (2020) | China | Cohort study | 44.5 | 202 | Hepatic steatosis index (HSI = 8 × [ALT/ AST] + BMI [+2 if type 2 diabetes yes, +2 if female]) >36 points and/or by abdominal ultrasound examination | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity | Good |
| 38 | Kim et al (2020) | US | Cohort study | 56.9 | 847 | Previous medical records | NA | Mortality | Good |
| 20 | Lee et al (2020) | South Korea | Cohort study | 61 | 1,005 | Laboratory investigations | ICU admission | Severity and mortality | Good |
| 40 | Lei et al (2020) | China | Cohort study | 56 | 5,771 | Previous medical records | Patients with respiratory rate >30 breaths/min, SpO2 <93% | Severity | Fair |
| 26 | Li et al (2020) | China | Cohort study | 59 | 104 | Laboratory investigations | NA | Mortality | Poor |
| 52 | Mahamid et al (2020) | Israel | RCS | 51 | 71 | Radiologic examination | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity | Poor |
| 24 | Mallet et al (2021) | France | RCS | 70 | 259,110 | NA | Mechanical ventilation | Severity and mortality | Good |
| 44 | Mushtaq et al (2020) | Qatar | Case-control study | NA | 589 | HSI index of 36 and above | NA | Severity and mortality | Poor |
| 39 | Navarathnam et al (2021) | England | RCS | NA | 91,541 | Previous medical records | NA | Mortality | Good |
| 45 | Posso et al (2020) | Spain | RCS | 78.2 | 834 | Previous medical records | NA | Mortality | Fair |
| 30 | Rodriguez-Gonzalez et al (2021) | Spain | Case-control study | 65 | 1,255 | Laboratory investigations | NA | Mortality | Fair |
| 41 | Sarin et al (2020) | 13 Asian countries | Cohort study | NA | 228 | Clinical and laboratory examination | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity and mortality | Poor |
| 46 | Schonfeld et al (2021) | Argentina | Cohort study | 42.9 | 207,079 | NA | ICU admission | Severity and mortality | Fair |
| 27 | Simon et al (2021) | Sweden | Cohort study | 60.9 | 224,467 | Liver biopsy | ICU admission | Severity | Good |
| 47 | Singh et al (2020) | US | Cohort study | NA | 2,780 | NA | NA | Mortality | Poor |
| 31 | Sun et al (2020) | China | Matched cohort study | 47 | 63 | Clinical and laboratory examination | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg; need for mechanical ventilation, ICU | Severity | Poor |
| 42 | Targher et al (2020) | China | Cohort study | NA | 310 | Laboratory investigations | ICU admission | Severity | Poor |
| 21 | de la Tijera et al (2021) | Mexico | Cross-sectional study | 50.6 | 166 | Previous medical records | Require invasive mechanical ventilation | Severity | Poor |
| 33 | Tobolowsky et al (2021) | US | Cohort study | 83 | 101 | NA | NA | Mortality | Poor |
| 22 | Veloz et al (2021) | Spain | Case- control study | NA | 447 | Historical medical records, radiology or analytic records within the last 24 months | NA | Mortality | Poor |
| 25 | Wang et al (2020) | China | Cohort study | 69 | 339 | Previous medical records | NA | Mortality | Good |
| 48 | Wang et al (2021) | US | RCS | 16,960 | Previous medical records | NA | Mortality | Poor | |
| 32 | Yang et al (2020) | China | Cohort study | 55 | 495 | Laboratory investigations | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity | Poor |
| 34 | Zhang et al (2021) | China | Case- control study | 47.9 | 172 | Laboratory investigations | Patients with respiratory rate >30 breaths/min, SpO2 <93% or PaO2/FiO2 ≤300 mm Hg | Severity | Poor |
| 28 | Zhou et al (2020) | China | Cohort study | 42.1 | 110 | Previous medical records | COVID-19 management guidance 7th edition | Severity | Fair |
Abbreviations: NA, not available; PaO2/FiO2, oxygenation index; RCS, retrospective cohort study; SpO2, oxygen saturation.
Error processing SSI fileThe opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.