At a glance
- Actionable practices for hospitals to improve diagnoses including diagnostic reasoning, testing, and communication activities, thereby improving patient safety.
- The Core Elements of Hospital Diagnostic Excellence were created in consultation with our federal partners, the Agency for Healthcare Research and Quality and the Centers for Medicare & Medicaid Services. The framework was also informed by expert knowledge within the clinical and patient community.
Summary
Diagnostic excellence (DxEx) encompasses multiple practices to improve diagnosis, including ensuring that diagnostic tests are ordered, interpreted, communicated, and acted upon appropriately.1 Approaches to achieve DxEx are designed to prevent missed, delayed, and incorrect diagnoses while reducing unnecessary testing and overdiagnosis.123456789 Several approaches can enhance diagnostic excellence, including diagnostic stewardship—strategies to guide the optimal use and interpretation of tests, improving processes and systems to support clinical decision-making, and tracking and learning from patient safety events related to missed, delayed, or incorrect diagnoses.12345678910111213 Improving communication with patients, families, and caregivers and improving professional teamwork also improves diagnosis.
Although the concepts of diagnostic stewardship, overdiagnosis, and DxEx have evolved separately in recent years, they share similar goals of optimizing diagnosis and function synergistically in a DxEx program.
The "Core Elements of Hospital Diagnostic Excellence" describes the key elements and actions of programs to improve diagnosis in the hospital. DxEx programs should select diagnoses to prioritize, engage the right people, implement improvement strategies tailored to available resources, and continuously monitor change.
A DxEx program may also lead to cost savings through more efficient care. Programs require leadership from a physician and a laboratory and/or radiology testing expert supported by the hospital leadership, as well as a diverse team of professionals, including frontline clinicians and nurses, patient safety, quality improvement, informatics, and other disciplines. Engagement with patients, their families, and caregivers is essential to the success of these programs.
DxEx programs should be a hospital resource that supports clinical decision-making, addresses challenges and concerns around diagnosis, and implements strategies to improve diagnosis. This document focuses on diagnostic excellence programs in acute care hospitals, but the same principles apply across the spectrum of care.
The Core Elements of Hospital Diagnostic Excellence Programs
Core Element
Description
1. Hospital Leadership Commitment and Accountability
Commitment to the staff and board that improving diagnosis is a priority for the hospital and ensuring the entire organization is accountable for progress.
Dedicating the necessary human, financial, technological, and information technology resources.
2. Multidisciplinary Expertise
Creating inclusive and multidisciplinary diagnostic teams that include laboratory and radiology testing experts.
3. Patient, Family, and Caregiver Engagement
Engaging patients, their families, and caregivers as partners in diagnostic excellence, including identifying effective ways to communicate diagnostic test results and other information.
4. Actions
Improving diagnosis through 1) diagnostic stewardship, 2) strengthening systems and processes, and 3) identifying, monitoring, and learning from diagnostic safety events.
Improving teamwork and coordination within the hospital and across the continuum of care.
5. Education
Educating healthcare personnel, patients, and family/caregivers about diagnosis and testing.
6. Tracking and Reporting
Monitoring and reporting the activities of the diagnostic excellence program.
Assessment tools and patient checklist
Introduction
Correct and timely diagnosis is essential for effective treatments and improving patient outcomes. Diagnosis is a multidisciplinary endeavor involving clinical decision-making between multiple clinicians in coordination with patients, families, and caregivers, involving laboratory, pathology, and radiological testing, all within a complex healthcare system. Failure to make an accurate and timely diagnosis is common and can be harmful, costly, and even fatal. The National Academies of Sciences, Engineering, and Medicine reports that most Americans will experience a diagnostic error in their lifetime.1 Diagnostic safety events (see Table 1) can lead to physical and psychological, emotional, social, and financial harm.123
The diagnostic process usually unfolds over time and in multiple locations of care. For instance, a patient may develop symptoms at home, look for answers on the internet, be assessed in the ambulatory setting, be referred to an emergency department, and then be admitted to a hospital. Moreover, diagnosing often involves uncertainty, and diagnoses evolve with time as information accrues. The diagnostic process consists of gathering and interpreting data from the patient's history, physical exam, testing, and consultations. Most hospitals have a patient safety and quality improvement department, but no group is dedicated to diagnostic excellence. For instance, there are few hospitals with teams to focus on diagnosis to ensure collaboration between clinicians and the laboratory or radiology department for optimal ordering, interpretation, and communication of diagnostic tests.4
The need for a program to support a complex and distributed care process within a hospital was also previously noted for antibiotic use and sepsis. In response, the Centers for Disease Control and Prevention (CDC) sought input from experts to develop the "Core Elements" of Antibiotic Stewardship" and subsequently applied the concept to sepsis programs.2425 The growing complexity and emphasis on diagnostic testing led the CDC to develop a similar approach to diagnostic excellence (DxEx) in this "Core Elements of Hospital Diagnostic Excellence Programs". Diagnostic excellence is broadly defined as making a correct and timely diagnosis using the fewest resources while maximizing patient experience and managing uncertainty.12 Several principles from antibiotic stewardship and sepsis core elements are also applicable here.
The Core Elements of DxEx Programs outline the structures and processes that hospitals can implement to improve the diagnostic process, including activities related to improving diagnostic reasoning, testing, and communication around diagnosis (see Figure 1). While these Core Elements are focused on hospital programs, similar principles apply to ambulatory medicine and other areas of healthcare.
DxEx programs can be effectively implemented in various hospitals and healthcare systems.10 The variability in hospital capacity and diverse patient populations served by hospitals requires flexibility in implementing DxEx programs. These Core Elements are informed by expert knowledge within the clinical and patient community, examination of peer-reviewed literature, and adaptation of features of effective quality improvement programs.
Figure 1
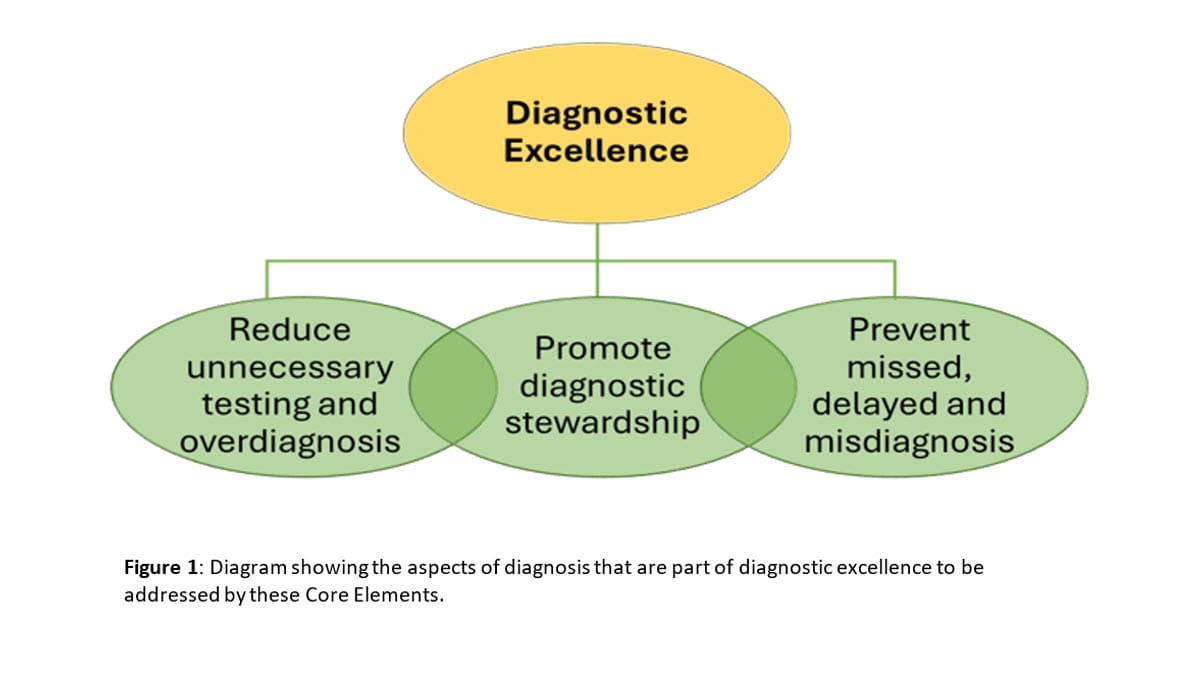
Table 1: Defining terms for improving diagnosis
This document uses several terms of importance for improving diagnosis, as described in Table 1. A DxEx program needs a holistic framework using balanced approaches that achieve accuracy and timeliness without excessive testing or overdiagnosis (see Figure 1).
Term related to diagnosis
Definition and/or example
Diagnostic Stewardship
Diagnostic Excellence
Making a correct and timely diagnosis using the fewest resources while maximizing patient experience and managing uncertainty.12
Overdiagnosis
Diagnosis of a condition that would never cause symptoms or harm to a patient.13
Unnecessary testing
Use of diagnostic tests that lack clear benefits or where harms outweigh benefits.7
Diagnostic testing capacity
Capacity and capability to perform necessary diagnostic tests in a timely and reliable manner.27
Diagnostic Safety Event
One or both of the following occurred, whether or not the patient was harmed:
- Delayed, wrong†, or missed diagnosis: One or more missed opportunities to pursue or identify an accurate and timely diagnosis (or other explanation) of the patient’s health problem(s) based on the information that existed at the time.
- Diagnosis not communicated to the patient: an accurate diagnosis (or other explanation) of the patient’s health problem(s) was available, but it was not communicated to the patient (includes the patient’s representative or family as applicable)
† Inappropriate diagnosis and misdiagnosis are other terms for wrong diagnosis.
While several definitions have been proposed and used to describe the diagnostic process and "diagnostic error," we use the Agency for Healthcare Research and Quality (AHRQ) definition of "diagnostic safety event" for this document913. The diagnostic process includes various steps in diagnosis where failures can occur, and interventions may be most effective (see Table 2).
Improving diagnosis through a DxEx program
DxEx programs can improve the quality of patient care and health outcomes.389111428293031 They can drive changes in culture, systems, and processes needed to optimize diagnosis. A DxEx program should serve as a central hub for patients, clinicians, laboratory professionals, and leadership, to address concerns about clinical practices and processes, technologies such as electronic health records (EHR), laboratory information systems (LIS), diagnostic tests, and other issues that impact diagnosis.
Testing can monitor or assess physiological status, response to therapy, and prognosis. This document primarily focuses on testing in the context of diagnostic evaluation in pursuit of a clinical diagnosis (not monitoring or other testing). The DxEx team can evaluate and recommend changes to hospital processes and promote initiatives to improve the use of diagnostic tests and diagnosis. The DxEx team may lead clinical, teaching, and quality improvement activities related to diagnosis. Clinicians are key to accurate diagnosis and should be engaged and supported by these programs.
CDC recommends that DxEx programs target problems in diagnosis that are frequent and preventable and where interventions are feasible and effective. For example, some programs could focus on improving the diagnosis of specific conditions such as pulmonary embolus or colorectal cancer, certain high-risk situations such as follow-up of chest imaging suspicious for lung malignancy or adopting diagnostic stewardship interventions for common infections. However, other organizations may decide to focus on disease-agnostic approaches to improve diagnostic safety (e.g., ensuring follow-up of test results pending at the time of hospital discharge to prevent missed diagnoses or clinical reasoning to improve test use); the focus of the program can be determined by the hospital DxEx team with input from other interested stakeholders. Specific program goals should be based on hospital needs, the organization's mission, the population it serves, and feedback from clinicians and patients.
Laboratory and radiology play an important role in the diagnostic process and improving diagnostic safety.32333435363738 Having timely access to relevant diagnostic tests is critical for diagnostic excellence. Diagnostic testing capacity at most US hospitals is generally well-developed, but this could pose challenges for hospitals with off-site labs, hospitals in low-resource settings, and hospitals in lower, middle-income countries (LMICs).27 Since the introduction of the Clinical Laboratory Improvement Amendments (CLIA) program39, significant reductions in error36 have been observed in the laboratory analytic phase of diagnostic testing, and work is ongoing to reduce pre-analytic and post-analytic errors. Similarly, there is a movement to reduce errors related to ordering and reporting in radiology.404142
DxEx is a multicomponent structured approach; all elements cannot be evaluated with the same evidence. Certain programmatic activities, mostly using diagnostic stewardship or physician cross-checking, have been evaluated in randomized controlled trials (RCTs)15161743 and large prospective quasi-experimental studies.1418192021 The outcomes of these activities include reducing misdiagnosis of catheter-associated urinary tract infection (CA-UTI), central line-associated bloodstream infection (CLABSI), and C. difficile infection by 30-60% with associated decreases in unnecessary antimicrobial use.821222331 Diagnostic stewardship approaches improve the diagnosis of pulmonary embolism through the pulmonary embolism rule-out criteria (PERC) and D-dimer testing.4445 Ordering approaches have reduced inappropriate head CT scans.46, MRI of the spine, and other testing.47 Reporting changes have improved the management of pulmonary nodules on imaging.48 Finally, even unnecessary daily laboratory testing in hospitalized patients has improved through diagnostic stewardship EHR interventions.4950 The individual interventions recommended have been found to be cost-effective or to reduce unnecessary testing and cascades of care, implying that significant savings are likely from the combined effect of these activities.182151
Table 2: The five domains of the diagnostic process and sub-components
Adapted from the Diagnostic Stewardship Pathway and Safer Dx Framework, the five domains can identify where failures occur and where interventions may be most effective. 3552
Domain 1
Clinician-patient encounter
- Effective communication between patients and caregivers
- Clinical reasoning
- Decisions about whether and what kind of diagnostic testing is needed
Domain 2
- Ordering tests, collecting, and transporting specimens (pre-analytic)
- Laboratory and radiology performance of tests (pre-, post, and analytic)
- Reporting and interpreting tests (post-analytic)
Diagnostic testing
Domain 3
Follow-up and tracking of diagnostic information
Domain 4
Consultation and coordination between specialists, other clinicians, and the clinical care team
Domain 5
Patient, family, and caregiver engagement throughout the diagnostic process
Program composition
A DxEx program will vary by the size of a hospital and the diagnoses that are the focus of the program. DxEx program leadership may initially come from existing antimicrobial stewardship programs, hospital epidemiology, quality, safety, or hospital medicine. Typically, a program will provide time for the following key positions dedicated to DxEx work:
- Physician co-lead (hospitalist, emergency medicine, patient safety researcher, etc.) The physician lead will oversee DxEx program activities, determine priority areas, and report efforts to leadership.
- Laboratory or radiology expert co-lead. Laboratory and radiology professionals on the team will provide knowledge about improving the use of diagnostic tests.
Leadership can ensure hospital-wide awareness and support of the DxEx program. At the minimum, DxEx programs will be more successful with strong support from clinicians, department or program heads, patient safety, quality improvement professionals, laboratory medicine committees, hospital epidemiology, antimicrobial stewardship, risk management, materials management, and nursing. Ideally, DxEx programs may also include staff from informatics and/or data analytics. DxEx programs should coordinate their activities and priorities with patient safety and quality improvement programs, but they are intended to function as stand-alone and independent programs to maintain a dedicated focus on diagnostic excellence.
CDC recommends that programs starting out initially focus on one or more priority examples (those with greater potential impact or a stronger evidence base) for each Core Element. More established programs can strive to achieve as many priority examples as possible and address other examples as well. Smaller hospitals or those in low-resource settings may focus on activities with a high return on their resource investments. For example, they can focus on diagnostic stewardship interventions for unnecessary tests that may be costly, such as molecular testing or advanced imaging. These savings may balance DxEx program costs.
Hospital Leadership Commitment and Accountability
Commitment and accountability from the hospital's senior leadership are essential and include support from the chief medical officer, chief quality or safety officer, chief nursing officer, and directors of pathology, laboratory services, and radiology. Achieving diagnostic excellence is consistent with the mission, values, and goals of all hospitals. Resource investments in DxEx programs, such as support for other safety aspects and clinical care delivery focused on improving quality and patient experience, are essential. The lack of necessary resources will be a barrier to the success of a DxEx program. Hospital leadership is critical in helping the DxEx program obtain the necessary resources to accomplish its goals. Hospital leadership can help ensure that other groups and departments know DxEx efforts and collaborate with the DxEx program.
Priority examples
- Commitment to the staff and board that improving diagnosis is a priority for the hospital and ensuring the entire organization is accountable for progress.
- Appointing one senior executive sponsor to serve as a point of contact or "champion" for the DxEx program to help ensure the program has the resources, capacity, and support to accomplish its mission.
- Providing at least two DxEx program co-leaders who are allocated time and resources to manage the program—a generalist physician lead (internal medicine, emergency medicine, etc.) and a laboratory and/or radiology testing expert.
- Requiring the following responsibilities from DxEx program leadership:
- Accountability for DxEx program management and outcomes
- Reporting of DxEx activities and outcomes (including key success stories) regularly to senior leadership and the hospital board.
- Accountability for DxEx program management and outcomes
- Monitoring the progress of the DxEx program to assess if the hospital is achieving its goals for improving diagnosis.
- Dedicating the necessary human, financial, technological, and information technology resources.
- Regular communication with staff and clinicians about the importance of DxEx and a commitment to a culture of safety that encourages reporting and learning from diagnostic safety events without blame.
Other examples
- Outlining DxEx-related duties in job descriptions and annual performance reviews for program leads and key support staff.
- Supporting only evidence-based diagnostic services and avoiding using testing as a revenue driver, e.g., do not adopt full-body MRI or "liquid biopsy" cancer screening tests without evidence of patient benefit.
- Integrating DxEx activities into other quality improvement and patient safety efforts.
- Having clear expectations for the program leaders for responsibilities and outcomes.
- Supporting training and education for program leaders and hospital staff (e.g., attending diagnosis-specific training courses and meetings).
- Supporting participation in local, state, and national DxEx quality improvement collaboratives.
- Ensuring staff from key support departments (outlined below) have sufficient time to contribute to DxEx activities.
- Assuring the support of key DxEx program members such as a quality/safety professional, a nurse, a data analyst, and an informatics expert.
Multidisciplinary Expertise
Achieving diagnostic excellence relies on many healthcare experts beyond clinicians. In addition to co-leadership, strong engagement from additional laboratory and radiology testing experts will make DxEx programs more successful. Most DxEx programs will include professionals with particular expertise in diagnostic testing, such as microbiologists, clinical chemists, surgical and clinical pathologists, and radiologists, depending on the specific diagnostic problem the hospital is addressing. Laboratory experts can work collaboratively to present data from lab reports in a way that supports DxEx and is consistent with hospital guidelines. Laboratory staff can guide discussions on the potential implementation of new diagnostic tests that may impact DxEx and collaborate with DxEx program personnel to develop guidance for clinicians when changes in laboratory testing practices might impact clinical decision-making.
Radiology staff can serve as consultants to improve the appropriate ordering of diagnostic imaging, aid in interpreting results, and optimize reporting to guide best practices. Additionally, radiology staff can guide discussions on the potential implementation of new diagnostic tests that may impact DxEx and collaborate with DxEx program personnel to develop guidance for clinicians when changes in radiology testing practices might impact clinical decision-making. Interpreting the combined output of diagnostic testing across the fields of imaging, pathology, clinical laboratory medicine, genomics, medical informatics, and information technology has been referred to as integrative diagnostics and may improve diagnosis.4053 Laboratory and radiology testing experts should engage key partners throughout the hospital and healthcare system. (See Actions for more specific changes.)
Advanced practice providers, nurses, pharmacists, psychologists, social workers, therapists, or technicians often spend more time with patients than physicians. They may have a better understanding of patient symptoms or disease course. These professionals are key members of a diagnostic team.54 These team members should be invited and encouraged to participate in efforts to improve diagnoses.54 Many other professions provide key support for a DxEx program, and the goals of each DxEx program should be determined as needed.
DxEx programs are greatly enhanced by strong support from the following groups:
- Regardless of their specialty, physicians must be engaged in and supportive of efforts to improve diagnosis. Generalists, including hospitalists, intensivists, and emergency medicine physicians, are especially important to engage because they make a large share of hospital diagnoses. However, all clinical areas and services should be invited to contribute to the program's work.
- Nurses can play an especially important role in optimizing testing or diagnostic stewardship. For example, nurses can inform decisions about whether a patient has symptoms that might justify reconsidering a current diagnosis.55 Nurses can also play key roles in diagnosis by recognizing changes in conditions and deteriorating health status, monitoring the timeliness of the diagnostic process, and using tools like TeamSTEPPS to help ensure effective communication between the patient and staff.555657
- Pharmacists, often thought of in relation primarily to medication dispensing, can actively participate in clinical teams, supporting diagnostic decision-making and ordering and interpreting diagnostic testing. Indication-based prescribing can improve DxEx52. Pharmacists are also experts in recognizing medication interactions and side effects that may be relevant to diagnosis.
- Allied Health Professionals, including medical technologists, occupational therapists, respiratory therapists, and speech pathologists, play a role in understanding DxEx and promoting optimal diagnosis.
- Pathology and laboratory staff can guide the proper use of tests and interpretation of results as part of diagnostic stewardship.
- Radiologists and radiology staff can guide the proper use and performance of radiological imaging.
- Department or program heads, including clinical department heads and the directors of laboratory services and radiology, can support embedding DxEx activities in daily practice.
- Laboratory medicine committee can play a key role in helping to develop and implement policies that will improve DxEx.
- DxEx leadership can emphasize the importance of diagnostic excellence when contracting for services, such as with offsite labs.
- Infection preventionists, hospital epidemiologists, and the antimicrobial stewardship team can assist with diagnostic stewardship efforts and DxEx around infectious disease diagnosis. They often have experience implementing diagnostic stewardship, including educating staff and analyzing intervention outcomes.
- Quality improvement and patient safety professionals can advocate for adequate resources and integrate DxEx interventions into other quality improvement efforts. They may also support implementation and outcome assessments.
- Risk management professionals can share and guide diagnosis-related issues that arise through medico-legal or other risk management activities. Risk management can also review and support efforts to improve diagnostic excellence.
- Information technology professionals are critical in integrating DxEx interventions into existing workflows. Some examples include embedding relevant information and protocols at the point of care (e.g., order sets and facility-specific guidelines), implementing clinical decision support for DxEx, and facilitating and maintaining DxEx reporting. Also, data analysts and informatics can perform measurements of testing rates, diagnoses and outcomes such as diagnostic safety events. Experts in AI and large language models may increasingly be needed in hospitals and DxEx programs.
- Genetic counselors often develop approaches to engaging patients and families in informed decisions about diagnostic testing. These skills can support a DxEx program in areas traditionally covered by genetic counseling, such as genetic testing, prenatal screening, and other diagnostic test decisions. Genetic counselors may also participate in diagnostic stewardship initiatives for genetic testing orders.
Priority examples
- Creating a diagnostic excellence program that is inclusive and multidisciplinary and includes laboratory and radiology testing experts.
- Laboratory and radiology testing experts facilitate understanding of commonly used tests from both the laboratory or radiological perspective (analytical validity) and the clinical interpretation of those tests in patients (clinical validity) and emphasize the difference between them.58
- Laboratory and radiology testing experts are involved in any changes to the use of common diagnostic tests within a laboratory, such as implementing sequential testing with an initial test followed by more specific tests to decrease false-positive results and overdiagnosis.1159
- Laboratory and radiology staff can work with DxEx program personnel to design optimal approaches to implement new diagnostic tests.
- Facilitate interaction between clinicians and laboratory and radiology testing experts through planned activities such as Tumor Boards60; Infectious Disease Laboratory Rounds where clinical and laboratory findings for a particular patient can be collated and discussed; routine laboratory-initiated clinician visits to the laboratory; or a clinical department inviting a laboratory and/or radiology professional to participate in clinical case discussions.
- Laboratory professionals are accessible in real-time for consultation regarding test selection and interpretation.
Patient, Family, and Caregiver Engagement
Open and effective communication with patients and their families is vital for diagnosis. This helps clinicians understand a patient’s illness and ensures patient understanding of their diagnosis.61 Discussing potential uncertainty in diagnosis as a natural part of the process is important.6263 Patient, family, and caregiver engagement is also central to shared decision-making. Patient, family, and caregiver groups can provide useful input to understand patient priorities for diagnostic excellence and help guide the DxEx program's goals.
A. Engage patients and families as partners in diagnostic decision-making.
Priority examples
- Facilitate patient direct access to diagnostic test results and their plain-language interpretations.
- Routinely seek feedback from patients and families to improve the explanation or communication of test results with appropriate literacy levels for patient interpretations.6465
- Strengthen patient, family, and caregiver engagement as partners in the diagnostic team through active listening and enhanced communication during clinician-patient encounters.66 This includes providing education and resources on explaining diagnostic uncertainty, orienting patients to the purpose of testing, and outlining how test results will be followed up.
- Use proactive approaches to ensure that patients, families, or caregivers understand discharge diagnoses and pending activities such as tests and consultations to improve review and action by outpatient physicians.5
Other examples
- Implement processes and tools to support shared decision-making in the diagnostic process.67
- Include patients and families in advisory committees related to quality and patient safety and ensure diversity in their representation.
- Provide patients with tools to help them communicate complete and accurate health information to the care team.686970
- Provide information about pending test results at the time of discharge, instructions for obtaining those results, and whom to contact if the results are not provided.
- Provide explanations of the relevance of key diagnostic tests (e.g., AST, ALT relate to liver disease).
B. Encourage patients and families to monitor changes in health and health information and bring forward concerns and questions about diagnosis.
Priority examples
- Provide access to health information, such as accessing portals using the patient's preferred device (i.e., smartphone, personal- or hospital-owned tablet).
- Increase patient, family, and caregiver engagement in the use of portals and address barriers to their use, such as language or internet access, or guide patients and families through how to set up and access portals.
- Ensure that patients, their families, and caregivers receive a list of any pending test results upon discharge, along with written instructions on how to obtain the results.717273
Other examples
- Facilitate review of records and test results in the portal.
- Create safe and easy systems for patients, families, or caregivers to report diagnostic safety events.
- Include questions that assess concerns about diagnosis in patient experience surveys.
C. Respond to patient, family, and caregiver concerns.
Priority examples
- Provide multiple channels through which patients and families can report diagnostic safety events and concerns, including a mechanism to initiate a review of their care.
- Have a formal process in place to investigate and respond to patient-reported diagnostic safety events and concerns (e.g., patient concerns about a missed diagnosis).
- Implement a mechanism to correct the medical record when patients identify incorrect information.74
Other examples
Actions
Interventions to improve diagnosis are critical to a DxEx program. The other Core Elements are program components necessary to undertake these Actions.
Three broad approaches to improving diagnosis are recommended here. The first involves the evidence-based implementation and use of diagnostic testing, known as diagnostic stewardship. The second involves strengthening systems and processes to improve accurate and timely diagnosis. The third involves surveillance for diagnostic safety events to learn from them and use this information to improve systems and processes. The examples provided are not exhaustive but are meant to illustrate how these approaches can enhance the diagnostic process of various conditions. They are organized into priority examples that all programs should consider and other examples for more established DxEx programs.
CDC recommends that all Actions be implemented using a multidisciplinary approach with the input of all relevant partners (laboratory professionals, safety/quality professionals, clinician end-users, information technology, infection control, leadership, etc.).38912 Examples of DxEx programs can be found in Measure Dx or other resources.4
A. Diagnostic stewardship
Diagnostic stewardship is the application of specific and evidence-based actions that lead clinicians to the right test for the right patient, leading to better interpretation and treatment decisions. This is achieved by collaborating with laboratory professionals to appropriately order or request testing, especially newer testing, improve laboratory utilization and performance, and interpret and report the results of diagnostic tests.389111429303176 These phases have also been described as the pre-analytic, analytic, and post-analytic steps (see Table 2). It should be noted that the laboratory term analytic specifically refers to highly standardized and regulated steps related to test performance.77 Some elements of laboratory processing in clinical diagnostic stewardship are considered pre-analytic by laboratory experts (e.g., requiring the presence of white blood cells (WBC) before performing a specific diagnostic test). Diagnostic stewardship typically provides a mechanism for clinicians to specially request tests (i.e., speak with the lab directly).
Diagnostic stewardship measurably improves patient care. It is used in many hospitals for infectious diseases and is advanced by the CDC.39
It has been applied to other common tests, including chemistry tests and radiological studies such as computerized tomography of the chest or magnetic resonance imaging of the spine.49 Diagnostic stewardship changes can have a larger impact than treatment-based interventions.1This concept is scalable because it is often associated with changes in the EHR or clinical laboratory. Thus, changes made once in the lab or EHR will impact the entire healthcare system.
Priority examples
- Ensure access to critical diagnostic tests as a part of laboratory capacity.78
- Restrict duplicate/repeat orders for tests with little additional benefit (C. difficile PCR, multiplex PCR tests, etc.).21317980
- Focus on appropriate specimen collection and handling to prevent errors (blood cultures, urine cultures, etc.).
- Improve diagnostic stewardship for frequently misused tests to optimize use. Initial targets could include urine cultures, C. difficile tests, blood cultures, respiratory cultures, and multiplex molecular panel tests.3225981828384
- Implement changes to test ordering, including:
- Require clinicians to document symptoms/indications when ordering urine cultures.5981
- Implement criteria for C. difficile testing (diarrhea, absence of laxatives).85
- Train clinicians in the appropriate reasons for blood culture ordering and methods for collection.22
- Assure protocols for pulmonary embolism testing that include identifying and managing low-risk patients, e.g., pulmonary embolism rule-out criteria (PERC) and D-dimer testing.4445
- Implement appropriateness criteria for ordering specific CT or MRI testing.4647
- Require clinicians to document symptoms/indications when ordering urine cultures.5981
- Implement changes to laboratory test processing such as:
- Implement changes to test reporting to improve care and reduce false positive diagnoses and subsequent overtreatment (e.g., when multiple organisms in urine culture are found, the report notes "likely contamination"; noting blood culture contamination; noting antibiotic susceptibilities; use standard reporting language for pulmonary nodules on chest imaging, etc.).
Other examples
- Improve the diagnostic pathway to optimize the use of other laboratory tests or diagnostic imaging, including daily labs49, troponin testing, or chest CT scans.
- Require laboratory expert review of orders for complex tests such as human genetic testing or pathogen metagenomic testing prior to completion.88
- Provide access to measures of test accuracy that support clinical reasoning, such as diagnostic sensitivity and specificity or population-specific positive or negative predictive values over analytic laboratory measures alone.58
- Provide easy access to reference materials, checklists, and resources for diagnosis (testingwisely.com and Global Knowledge Sharing Platform for Patient Safety)
- Provide comprehensive menus of diagnostic tests online or linked to the EHR and laboratory website for easy accessibility.
B. Strengthen systems and processes to support accurate and timely diagnosis.
Clinicians make diagnoses within complex healthcare systems. Breakdowns occur when patient information is not gathered or interpreted adequately, needed tests are not ordered, tests are lost in transit, unnecessary tests are ordered, test results fail to reach the responsible clinician, responsibility for follow-up is ambiguous, and important diagnostic information is not communicated or shared effectively. Programs can actively map the process of tests from decisions to order all the way to how the patient or caregiver is provided the results to determine vulnerabilities and potential interventions.89 One way to mitigate testing breakdowns is to establish standards for communicating test results. Several strategies have been suggested to mitigate breakdowns related to information gathering and interpretation, including encouraging clinicians to review and reflect on their own diagnostic performance.1290 Clinicians can manage and communicate diagnostic uncertainty to patients and/or initiate a "diagnostic time out" in the right situations. They can also seek help from other clinicians, specialist experts, or diagnostic decision-support tools when there are questions about the diagnosis. Promoting safe environments to discuss diagnostic safety events without blame for individual clinicians is essential.
Priority examples
- Identify opportunities to improve teamwork and coordination within the hospital and continuum of care.
- Provide training and protocols for communicating diagnostic uncertainty during transitions of care.91
- Ensure that diagnostic findings are reliably transmitted to the clinician responsible for follow-up through closed-loop communication92 or other methods (using resources such as the SAFER Guide for Test Results Reporting and Follow-up). Clarify responsibility and process for following up on abnormal clinical findings. Several healthcare systems have policies that ensure that ordering clinicians are responsible for follow-up of results of tests they order unless there are preexisting, mutually agreeable, and clear arrangements made with a designee (VHA Directive 1088 Communicating Test Results to Providers and Patients).
- Ensure that diagnostic specialties are included in the development of clinical care pathways and standards related to diagnostic evaluation.
Other examples
- Implement processes for all tests that include tracking procedures to ensure fail-safe communication.
- Develop a process map to determine vulnerabilities related to the total testing process from test ordering to results delivery to patients.89
- Implement policies and standardized workflows for communication of critical test results93 and assess adherence over time.
- Develop and formalize a "diagnostic time out"94 process for clinicians and patients experiencing prolonged uncertainty in diagnosis.
C. Identifying and learning from diagnostic safety events.
Clinicians are a resource for information about diagnosis. However, safety events, including diagnostic safety events, are often not reported.95 Clinicians should be encouraged and/or incentivized to report diagnostic safety events. Many safety events are recorded by quality and safety staff as part of their routine monitoring. Those that are diagnostic safety events (e.g., delays in diagnosis of sepsis, misdiagnosis of catheter-associated urinary tract infection (CAUTI)) could be reviewed and addressed by the DxEx team.
More formalized and rigorous methods can be used to identify diagnostic safety events using electronic trigger tools and standardized review instruments.96 Tools, including AHRQ's Measure Dx resource, can guide the identification of diagnostic safety events. The diagnostic problems identified by these reviews should then lead to changes in clinician or hospital practice to prevent these events by addressing steps in the diagnostic process. Techniques, such as using EHR data to identify diagnostic safety concerns using electronic triggers,96 are ready for implementation. In the future, advanced analytic techniques using machine learning and generative artificial intelligence may also be useful for this purpose.
Priority examples
- Identify diagnostic safety events from routine quality and safety monitoring.979899100101
- Provide mechanisms for clinicians and staff to bring forward concerns about diagnosis that can be further investigated by the DxEx team.
Other examples
- Perform surveillance of EHR data to identify records of patients who are at high risk for diagnostic safety events (for example, use tools such as electronic triggers followed by medical record reviews using the Revised Safer Dx Instrument).
- Use aggregated diagnostic safety data to identify patterns that suggest areas of improvement, including inequities.
- Implement actions and interventions based on lessons learned from diagnostic safety events and ensure their sustainability.
- Provide feedback to involved clinicians and team members about diagnostic processes (e.g., test use) and outcomes (e.g., feedback and monitoring for mismatch between test order and clinical question or feedback on diagnostic safety events).
- Review of key points learned from diagnostic safety events during clinical conferences, such as Grand Rounds and Tumor Boards.
- Conduct a risk assessment of commonly misdiagnosed high-risk conditions in the ED (or other high-risk settings).
Education
Education is important to improving DxEx by promoting better clinical reasoning.12 However, while most actions to improve diagnosis have an element of education, more than education is needed to improve DxEx. Education should emphasize recognizing problems that lead to diagnostic safety events. This knowledge provides the opportunity to rethink care and improve diagnostic outcomes. Ideally, this would be part of a broader Learning Healthcare System.102
Clinical reasoning is the cognitive process of identifying and prioritizing clinical information to formulate diagnoses and make other clinical decisions.103 Clinical reasoning involves appropriately weighing information, such as the impact of different symptoms and risk factors and avoiding irrational use of information based on biases. One way to improve clinical reasoning is to educate clinicians, laboratory experts, nurses, and patients about the diagnostic process and the value of diagnostic testing. This includes estimating the probability of different diseases and adjusting for new information, such as diagnostic test results and thresholds for testing or treatment.104105106 It also includes awareness of clinical cascades that can occur due to testing,107108 and how cognitive biases, such as overconfidence and anchoring, can impact accurate diagnosis and the value of taking a diagnostic 'time out' to review their conclusions. 109110111112Clinicians also need to recognize that implicit biases, for example, based on race, can influence the diagnostic process.113114115116117118119120121
Priority examples
- Ensure patients and family/caregivers understand the important role of diagnosis and testing.122123124125
- Promote understanding of clinical reasoning and probability to students, trainees, clinicians, and hospital leaders.126127
- Inform clinicians about thresholds for testing and treatment decisions.104
- Ensure that clinicians are provided clinical reasoning aids and other knowledge resources for diagnosis (e.g., CDC disease-specific guidance, specialty guidelines, testingwisely.com).
- Provide current best practices for understanding and communicating diagnostic uncertainty when discussing with patients128 and during the transition of care.
- Provide education about cognitive biases and situations where biases based on patient characteristics (e.g., race) and co-occurring conditions are likely to be more common.
- Improve feedback by tracking patient outcomes and/or seeking feedback directly from peers or patients and families.12
- Encourage clinicians to implement reflective practices (e.g., periodically review patients' diagnostic outcomes and reflect on one's reasoning and decisions, consider using AHRQ's Calibrate Dx: A Resource to Improve Diagnostic Decisions).5
Other examples
- Consider simulation-based training to improve feedback on diagnostic accuracy.5
- As a part of clinical reasoning, educate clinicians about the value of evaluating and learning from diagnostic safety events.
- Educate providers how to leverage the knowledge and skills of other professionals on the care team when making a diagnosis.
- Provide education reflective practice skills, including a healthy skepticism of certainty in diagnosis and reconsidering diagnosis when patients do not respond to therapy as expected.
- Educate clinicians about overdiagnosis and incidental findings as harms from screening or diagnostic testing.
- Orient learners to resources that can be used for regular, brief skill-building in diagnostic reasoning (e.g., brief digital learning activities).12
Tracking and Reporting
Surveillance activities such as tracking, reporting, and monitoring are important tools for quality improvement, identifying problems, and the impact of interventions. However, there are no broadly used metrics of diagnostic safety events that would be suitable for comparing hospital performance. DxEx programs should develop internal program-specific metrics to evaluate progress toward their goals. These metrics should align with the actions that the DxEx program is addressing.
A. Diagnostic stewardship tracking is most developed for infectious disease tests and diagnoses but can be modified by DxEx program goals.
Priority examples
- Track the presence and effectiveness of diagnostic stewardship interventions to improve diagnosis of UTI, C. difficile infection, and bloodstream infections.
Other examples
- Monitor types of diagnostic imaging testing if they are a target of diagnostic stewardship (e.g., MRI of the spine, CT chest) and, if possible, rates of inappropriate use.
B. Strengthen systems and processes to support accurate and timely diagnosis.
Priority examples
- Report the presence of forums to discuss challenging cases (e.g., M&M conferences, tumor boards, or case conferences).
- Report which disease conditions or high-risk situations are being targeted for improvement efforts to strengthen systems and processes (for instance, learning from safety events may reveal several cases of missed spinal epidural abscesses that require process improvements).
- Report specific actions and interventions that were taken to address the process of care or system gaps and opportunities to achieve diagnostic excellence.
Other examples
- Laboratories examine and report turnaround time (TAT) for commonly used tests.
- Monitor and intervene in instances of test results reported to the wrong clinician, a clinician who has left the service, or not received by the clinician following the patient.
- Track receipt and action taken on recommendations for follow-up of incidental imaging findings.129
C. Identifying and learning from diagnostic safety events. Identify reliable and valid data sources. Measurement must focus on areas with strong potential for learning and impact.
Priority examples
- Report frequencies and types of diagnostic safety events identified from routine quality and safety monitoring.979899100101
Other examples
- Track the number of diagnostic safety concerns reported by clinicians and patients.97130131132
- Identify the frequency of diagnostic safety events using electronic trigger tools followed by reviews of medical records using Revised Safer Dx Instrument.97133134135
- Track the number of root cause analysis on diagnostic safety events that identified problems needing correction.
- Track patient-reported diagnostic concerns over time to understand trends.74
Resources
Diagnostic stewardship
- CDC White Paper on Diagnostic Stewardship to improve diagnostic safety events.
- Principles of Diagnostic Stewardship by the Society for Healthcare Epidemiology of America (SHEA), with endorsement from the Infectious Disease Society of America (IDSA), Society for Hospital Medicine (SHM), Society for Infectious Disease Pharmacists (SIDP), and Association for Professionals in Infection Control and Epidemiology (APIC).
- The relationship between diagnostic stewardship and antimicrobial stewardship by SHEA, IDSA, SHM, SIDP & APIC.
- Diagnostic Stewardship and healthcare-associated infection (HAI) metrics by SHEA, IDSA, SHM, SIDP & APIC.
- Diagnostic stewardship for emerging pathogens by SHEA, IDSA, SHM, SIDP & APIC.
Trainings and toolkits
- Tools to Improve Diagnosis from Society to Improve Diagnosis in Medicine (SIDM)
- Animations to teach clinical reasoning, diagnostic calculators, and BIRD game for Bayesian updating of test results
- Safer Dx Checklist: 10 organizational practices for diagnostic excellence
- Measure Dx: a resource to identify, analyze, and learn from diagnostic safety events
- Calibrate Dx: a resource to improve diagnostic decisions
- Team STEPPS: a diagnosis improvement course
- Improving Your Laboratory Testing Process: a guide for office diagnostic testing
Patient tools
Clinical Laboratory Improvement Amendments (CLIA)
- National Academies of Sciences, Engineering, and Medicine. 2015. Improving diagnosis in health care. Washington, DC: The National Academies Press. https://nap.nationalacademies.org/catalog/21794/improving-diagnosis-in-health-care.
- Korenstein D, Harris R, Elshaug AG, et al. To Expand the Evidence Base About Harms from Tests and Treatments. J Gen Intern Med. 2021;36(7):2105-2110. doi:10.1007/s11606-021-06597-9
- Fabre V, Davis A, Diekema DJ, et al. Principles of diagnostic stewardship: A practical guide from the Society for Healthcare Epidemiology of America Diagnostic Stewardship Task Force. Infect Control Hosp Epidemiol. 2023;44(2):178-185. doi:10.1017/ice.2023.5
- Giardina TD, Shahid U, Mushtaq U, Upadhyay DK, Marinez A, Singh H. Creating a Learning Health System for Improving Diagnostic Safety: Pragmatic Insights from US Health Care Organizations. J Gen Intern Med. 2022;37(15):3965-3972. doi:10.1007/s11606-022-07554-w
- Singh H, Zwaan L. Web Exclusives. Annals for Hospitalists Inpatient Notes - Reducing Diagnostic Error-A New Horizon of Opportunities for Hospital Medicine. Ann Intern Med. 2016;165(8):HO2-HO4. doi:10.7326/M16-2042
- Pierce VM, Simner PJ, Lonsway DR, et al. Modified Carbapenem Inactivation Method for Phenotypic Detection of Carbapenemase Production among Enterobacteriaceae. J Clin Microbiol. 2017;55(8):2321-2333. doi:10.1128/JCM.00193-17
- O'Sullivan JW, Albasri A, Nicholson BD, et al. Overtesting and undertesting in primary care: a systematic review and meta-analysis. BMJ Open. 2018;8(2):e018557. doi:10.1136/bmjopen-2017-018557
- Morgan DJ, Malani PN, Diekema DJ. Diagnostic Stewardship to Prevent Diagnostic Error. JAMA. Published online March 2, 2023. doi:10.1001/jama.2023.1678
- Curren EJ, Lutgring JD, Kabbani S, et al. Advancing Diagnostic Stewardship for Healthcare-Associated Infections, Antibiotic Resistance, and Sepsis. Clin Infect Dis. 2022;74(4):723-728. doi:10.1093/cid/ciab672
- Recognizing Excellence in Diagnosis: Recommended Practices for Hospitals | Leapfrog. Accessed April 25, 2024. https://www.leapfroggroup.org/recognizing-excellence-diagnosis-recommended-practices-hospitals
- Claeys KC, Zhan M, Pineles L, et al. Conditional reflex to urine culture: Evaluation of a diagnostic stewardship intervention within the Veterans' Affairs and Centers for Disease Control and Prevention Practice-Based Research Network. Infect Control Hosp Epidemiol. 2021;42(2):176-181. doi:10.1017/ice.2020.400
- Singh H, Connor DM, Dhaliwal G. Five strategies for clinicians to advance diagnostic excellence. BMJ. 2022;376:e068044. doi:10.1136/bmj-2021-068044
- Brodersen J, Schwartz LM, Heneghan C, O'Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn't. BMJ Evid Based Med. 2018;23(1):1-3. doi:10.1136/ebmed-2017-110886
- Epstein L, Diekema DJ, Morgan DJ, et al. Diagnostic stewardship and the coronavirus disease 2019 (COVID-19) pandemic: Lessons learned for prevention of emerging infectious diseases in acute-care settings. Infect Control Hosp Epidemiol. Published online November 7, 2023:1-7. doi:10.1017/ice.2023.195
- Pasay DK, Guirguis MS, Shkrobot RC, et al. Antimicrobial stewardship in rural nursing homes: Impact of interprofessional education and clinical decision tool implementation on urinary tract infection treatment in a cluster randomized trial. Infect Control Hosp Epidemiol. 2019;40(4):432-437. doi:10.1017/ice.2019.9
- Markussen DL, Serigstad S, Ritz C, et al. Diagnostic Stewardship in Community-Acquired Pneumonia With Syndromic Molecular Testing: A Randomized Clinical Trial. JAMA Netw Open. 2024;7(3):e240830. doi:10.1001/jamanetworkopen.2024.0830
- Banerjee R, Teng CB, Cunningham SA, et al. Randomized Trial of Rapid Multiplex Polymerase Chain Reaction-Based Blood Culture Identification and Susceptibility Testing. Clin Infect Dis. 2015;61(7):1071-1080. doi:10.1093/cid/civ447
- Lynch CS, Appleby-Sigler A, Bork JT, et al. Effect of urine reflex culturing on rates of cultures and infections in acute and long-term care. Antimicrob Resist Infect Control. 2020;9(1):96. doi:10.1186/s13756-020-00762-1
- Watson KJ, Trautner B, Russo H, et al. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: A multicenter experience. Infect Control Hosp Epidemiol. 2020;41(5):564-570. doi:10.1017/ice.2020.37
- Leis JA, Rebick GW, Daneman N, et al. Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi:10.1093/cid/ciu010
- Rock C, Perlmutter R, Blythe D, et al. Impact of Statewide Prevention and Reduction of Clostridioides difficile (SPARC), a Maryland public health-academic collaborative: an evaluation of a quality improvement intervention. BMJ Qual Saf. 2022;31(2):153-162. doi:10.1136/bmjqs-2021-014014
- Woods-Hill CZ, Colantuoni EA, Koontz DW, et al. Association of Diagnostic Stewardship for Blood Cultures in Critically Ill Children With Culture Rates, Antibiotic Use, and Patient Outcomes: Results of the Bright STAR Collaborative. JAMA Pediatrics. 2022;176(7):690-698. doi:10.1001/jamapediatrics.2022.1024
- Vaughn VM, Gupta A, Petty LA, et al. A Statewide Quality Initiative to Reduce Unnecessary Antibiotic Treatment of Asymptomatic Bacteriuria. JAMA Intern Med. 2023;183(9):933-941. doi:10.1001/jamainternmed.2023.2749
- Centers for Disease Control and Prevention. Core Elements of Antibiotic Stewardship. September 7, 2023. https://www.cdc.gov/antibiotic-use/core-elements/index.html
- Hospital Sepsis Program Core Elements | Sepsis | CDC. January 29, 2024. Accessed February 5, 2024. https://www.cdc.gov/sepsis/core-elements.html
- Morgan DJ, Malani P, Diekema DJ. Diagnostic Stewardship-Leveraging the Laboratory to Improve Antimicrobial Use. JAMA. 2017;318(7):607-608. doi:10.1001/jama.2017.8531
- World Health Organization. Diagnostic Stewardship: A Guide to Implementation in Antimicrobial Resistance Surveillance Sites.; 2016. Accessed June 20, 2024. https://www.who.int/publications/i/item/WHO-DGO-AMR-2016.3
- PSOPPC: Diagnostic Safety 1.0. Accessed January 2, 2024. https://www.psoppc.org/psoppc_web/publicpages/commonFormatsDSV1.0
- Morgan DJ, Leekha S, Claeys KC. Increasing Evidence That Diagnostic Stewardship May Improve Antibiotic Use. JAMA Intern Med. 2023;183(9):942-943. doi:10.1001/jamainternmed.2023.2756
- Ku TSN, Al Mohajer M, Newton JA, et al. Improving antimicrobial use through better diagnosis: The relationship between diagnostic stewardship and antimicrobial stewardship. Infect Control Hosp Epidemiol. 2023;44(12):1901-1908. doi:10.1017/ice.2023.156
- Rock C, Abosi O, Bleasdale S, et al. Clinical Decision Support Systems to Reduce Unnecessary Clostridioides difficile Testing Across Multiple Hospitals. Clin Infect Dis. 2022;75(7):1187-1193. doi:10.1093/cid/ciac074
- Itri JN, Tappouni RR, McEachern RO, Pesch AJ, Patel SH. Fundamentals of Diagnostic Error in Imaging. Radiographics. 2018;38(6):1845-1865. doi:10.1148/rg.2018180021
- Heher YK, Chen Y, VanderLaan PA. Pre-analytic error: A significant patient safety risk. Cancer Cytopathol. 2018;126 Suppl 8:738-744. doi:10.1002/cncy.22019
- Higgins MCSS, Herpy JP. Medical Error, Adverse Events, and Complications in Interventional Radiology: Liability or Opportunity? Radiology. 2021;298(2):275-283. doi:10.1148/radiol.2020202341
- Plebani M. Exploring the iceberg of errors in laboratory medicine. Clin Chim Acta. 2009;404(1):16-23. doi:10.1016/j.cca.2009.03.022
- Hammerling JA. A Review of Medical Errors in Laboratory Diagnostics and Where We Are Today. Laboratory Medicine. 2012;43(2):41-44. doi:10.1309/LM6ER9WJR1IHQAUY
- Plebani M, Laposata M, Lundberg GD. The brain-to-brain loop concept for laboratory testing 40 years after its introduction. Am J Clin Pathol. 2011;136(6):829-833. doi:10.1309/AJCPR28HWHSSDNON
- Mrazek C, Lippi G, Keppel MH, et al. Errors within the total laboratory testing process, from test selection to medical decision-making - A review of causes, consequences, surveillance and solutions. Biochem Med (Zagreb). 2020;30(2):020502. doi:10.11613/BM.2020.020502
- CLIA Law & Regulation. November 14, 2022. Accessed April 29, 2024. https://www.cdc.gov/clia/law-regulations.html
- Beauchamp NJ, Bryan RN, Bui MM, et al. Integrative diagnostics: the time is now-a report from the International Society for Strategic Studies in Radiology. Insights Imaging. 2023;14(1):54. doi:10.1186/s13244-023-01379-9
- Bruno MA, Walker EA, Abujudeh HH. Understanding and Confronting Our Mistakes: The Epidemiology of Error in Radiology and Strategies for Error Reduction. Radiographics. 2015;35(6):1668-1676. doi:10.1148/rg.2015150023
- Degnan AJ, Ghobadi EH, Hardy P, et al. Perceptual and Interpretive Error in Diagnostic Radiology-Causes and Potential Solutions. Acad Radiol. 2019;26(6):833-845. doi:10.1016/j.acra.2018.11.006
- Freund Y, Goulet H, Leblanc J, et al. Effect of Systematic Physician Cross-checking on Reducing Adverse Events in the Emergency Department: The CHARMED Cluster Randomized Trial. JAMA Intern Med. 2018;178(6):812-819. doi:10.1001/jamainternmed.2018.0607
- Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev. 2016;2016(8):CD010864. doi:10.1002/14651858.CD010864.pub2
- Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi:10.1111/j.1538-7836.2008.02944.x
- Agency for Healthcare Research and Quality. When Costly and Potentially Harmful CT Scans Are Not Necessary | Digital Healthcare Research. Accessed April 24, 2024. https://digital.ahrq.gov/2018-year-review/research-summary/when-costly-and-potentially-harmful-ct-scans-are-not-necessary
- Larijani M, Azizan A, Carr T, Badea A, Groot G. Reducing Inappropriate Imaging Orders For Lower Back Pain Using MRI And CT Checklists: A Quality Improvement Study In Saskatchewan, Canada. Quality in Primary Care. 2020;28(4):24-31.
- Christensen J, Prosper AE, Wu CC, et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Chest. 2024;165(3):738-753. doi:10.1016/j.chest.2023.10.028
- Eaton KP, Levy K, Soong C, et al. Evidence-Based Guidelines to Eliminate Repetitive Laboratory Testing. JAMA Intern Med. 2017;177(12):1833-1839. doi:10.1001/jamainternmed.2017.5152
- Vaughn VM, Morgan DJ. Diagnostic stewardship: Improving use of diagnostic tests for better quality and value in hospital medicine. J Hosp Med. Published online March 13, 2024. doi:10.1002/jhm.13321
- Pliakos EE, Andreatos N, Shehadeh F, Ziakas PD, Mylonakis E. The Cost-Effectiveness of Rapid Diagnostic Testing for the Diagnosis of Bloodstream Infections with or without Antimicrobial Stewardship. Clin Microbiol Rev. 2018;31(3):e00095-17. doi:10.1128/CMR.00095-17
- Schiff GD, Seoane-Vazquez E, Wright A. Incorporating Indications into Medication Ordering — Time to Enter the Age of Reason. N Engl J Med. 2016;375(4):306-309. doi:10.1056/NEJMp1603964
- Lundström CF, Gilmore HL, Ros PR. Integrated Diagnostics: The Computational Revolution Catalyzing Cross-disciplinary Practices in Radiology, Pathology, and Genomics. Radiology. 2017;285(1):12-15. doi:10.1148/radiol.2017170062
- Graber ML, Rusz D, Jones ML, et al. The new diagnostic team. Diagnosis (Berl). 2017;4(4):225-238. doi:10.1515/dx-2017-0022
- Reinforcing the Value and Roles of Nurses in Diagnostic Safety: Pragmatic Recommendations for Nurse Leaders and Educators. Accessed January 2, 2024. https://www.ahrq.gov/patient-safety/reports/issue-briefs/nurse-role-dxsafety.html
- Guide to Improving Patient Safety in Primary Care Settings by Engaging Patients and Families. Accessed July 29, 2024. https://www.ahrq.gov/patient-safety/reports/engage.html
- Section 1: Overview of Key Concepts and Tools | Agency for Healthcare Research and Quality. Accessed July 29, 2024. https://www.ahrq.gov/teamstepps-program/curriculum/communication/overview/index.html
- Saah AJ, Hoover DR. "Sensitivity" and "specificity" reconsidered: the meaning of these terms in analytical and diagnostic settings. Ann Intern Med. 1997;126(1):91-94. doi:10.7326/0003-4819-126-1-199701010-00026
- Claeys KC, Trautner BW, Leekha S, et al. Optimal Urine Culture Diagnostic Stewardship Practice- Results from an Expert Modified-Delphi Procedure. Clin Infect Dis. Published online November 29, 2021:ciab987. doi:10.1093/cid/ciab987
- Okasako J, Bernstein C. Multidisciplinary Tumor Boards and Guiding Patient Care: The AP Role. J Adv Pract Oncol. 2022;13(3):227-230. doi:10.6004/jadpro.2022.13.3.9
- Huynh K, Brito JP, Bylund CL, Prokop LJ, Ospina NS. Understanding diagnostic conversations in clinical practice: A systematic review. Patient Educ Couns. 2023;116:107949. doi:10.1016/j.pec.2023.107949
- Meyer AND, Giardina TD, Khawaja L, Singh H. Patient and clinician experiences of uncertainty in the diagnostic process: Current understanding and future directions. Patient Educ Couns. 2021;104(11):2606-2615. doi:10.1016/j.pec.2021.07.028
- Dahm MR, Crock C. Understanding and Communicating Uncertainty in Achieving Diagnostic Excellence. JAMA. 2022;327(12):1127-1128. doi:10.1001/jama.2022.2141
- Vipler B. "What's Lymphoma?" - Risks Posed by Immediate Release of Test Results to Patients. N Engl J Med. 2024;390(12):1064-1066. doi:10.1056/NEJMp2312953
- Interoperability and Patient Access Fact Sheet | CMS. Accessed April 25, 2024. https://www.cms.gov/newsroom/fact-sheets/interoperability-and-patient-access-fact-sheet
- Toolkit for Engaging Patients To Improve Diagnostic Safety. Accessed September 4, 2024. https://www.ahrq.gov/diagnostic-safety/tools/engaging-patients-improve.html
- Berger ZD, Brito JP, Ospina NS, et al. Patient centred diagnosis: sharing diagnostic decisions with patients in clinical practice. BMJ. 2017;359:j4218. doi:10.1136/bmj.j4218
- Question Builder | Agency for Healthcare Research and Quality. Accessed September 4, 2024. https://www.ahrq.gov/questions/question-builder/online.html
- Ask Me 3: Good Questions for Your Good Health | Institute for Healthcare Improvement. Accessed September 4, 2024. https://www.ihi.org/resources/tools/ask-me-3-good-questions-your-good-health
- Toolkit for Engaging Patients To Improve Diagnostic Safety. Agency for Healthcare Research and Quality; 2021. Accessed September 4, 2024. https://www.ahrq.gov/sites/default/files/wysiwyg/patient-safety/resources/diagnostic-toolkit/10-diagnostic-safety-tool-patient-note-sheet.pdf
- Department of Veterans Affairs. VHA Directive 1088: Communicating Test Results to Providers and Patients. Published online July 11, 2023.
- Whitehead NS, Williams L, Meleth S, et al. Interventions to Improve Follow-Up of Laboratory Test Results Pending at Discharge: A Systematic Review. J Hosp Med. 2018;13(9):631-636. doi:10.12788/jhm.2944
- Darragh PJ, Bodley T, Orchanian-Cheff A, Shojania KG, Kwan JL, Cram P. A Systematic Review of Interventions to Follow-Up Test Results Pending at Discharge. J Gen Intern Med. 2018;33(5):750-758. doi:10.1007/s11606-017-4290-9
- Schlesinger M, Grob R, Gleason K, et al. Patient Experience as a Source for Understanding the Origins, Impact, and Remediation of Diagnostic Errors. Volume 1: Why Patient Narratives Matter. Agency for Healthcare Research and Quality (US); 2023.
- Communication and Optimal Resolution (CANDOR) Toolkit | Agency for Healthcare Research and Quality. Accessed September 4, 2024. https://www.ahrq.gov/patient-safety/settings/hospital/candor/modules.html
- Patel R, Fang FC. Diagnostic Stewardship: Opportunity for a Laboratory-Infectious Diseases Partnership. Clin Infect Dis. 2018;67(5):799-801. doi:10.1093/cid/ciy077
- 42 CFR 493.1289 -- Standard: Analytic systems quality assessment. Accessed April 25, 2024. https://www.ecfr.gov/current/title-42/part-493/section-493.1289
- Global Antimicrobial Resistance and Use Surveillance System (GLASS). Accessed April 25, 2024. https://www.who.int/initiatives/glass
- Morgan DJ, Pineles L, Owczarzak J, et al. Accuracy of Practitioner Estimates of Probability of Diagnosis Before and After Testing. JAMA Intern Med. 2021;181(6):747-755. doi:10.1001/jamainternmed.2021.0269
- Morgan DJ, Croft LD, Deloney V, et al. Choosing Wisely in Healthcare Epidemiology and Antimicrobial Stewardship. Infect Control Hosp Epidemiol. 2016;37(7):755-760. doi:10.1017/ice.2016.61
- Claeys KC, Weston LE, Pineles L, Morgan DJ, Krein SL. Implementing diagnostic stewardship to improve diagnosis of urinary tract infections across three medical centers: A qualitative assessment. Infect Control Hosp Epidemiol. 2023;44(12):1932-1941. doi:10.1017/ice.2023.106
- Baghdadi JD, O'Hara LM, Johnson JK, Krein SL, Harris AD, Morgan DJ. Diagnostic stewardship to support optimal use of multiplex molecular respiratory panels: A survey from the Society for Healthcare Epidemiology of America Research Network. Infect Control Hosp Epidemiol. 2023;44(11):1823-1828. doi:10.1017/ice.2023.72
- Sullivan KV, Gallagher JC, Leekha S, et al. Use of diagnostic and antimicrobial stewardship practices to improve Clostridioides difficile testing among SHEA Research Network hospitals. Infect Control Hosp Epidemiol. Published online August 11, 2021:1-5. doi:10.1017/ice.2021.133
- Kenaa B, Richert ME, Claeys KC, et al. Ventilator-Associated Pneumonia: Diagnostic Test Stewardship and Relevance of Culturing Practices. Curr Infect Dis Rep. 2019;21(12):50. doi:10.1007/s11908-019-0708-3
- Rock C, Maragakis LL. Diagnostic Stewardship for Clostridiodes difficile Testing: From Laxatives to Diarrhea and Beyond. Clin Infect Dis. 2020;71(6):1479-1480. doi:10.1093/cid/ciz982
- Epstein L, Edwards JR, Halpin AL, et al. Evaluation of a Novel Intervention to Reduce Unnecessary Urine Cultures in Intensive Care Units at a Tertiary Care Hospital in Maryland, 2011-2014. Infect Control Hosp Epidemiol. 2016;37(5):606-609. doi:10.1017/ice.2016.9
- Messacar K, Palmer C, Gregoire L, et al. Clinical and Financial Impact of a Diagnostic Stewardship Program for Children with Suspected Central Nervous System Infection. J Pediatr. 2022;244:161-168.e1. doi:10.1016/j.jpeds.2022.02.002
- Miller CE, Krautscheid P, Baldwin EE, et al. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. Am J Med Genet A. 2014;164A(5):1094-1101. doi:10.1002/ajmg.a.36453
- Murphy DR, Satterly T, Rogith D, Sittig DF, Singh H. Barriers and facilitators impacting reliability of the electronic health record-facilitated total testing process. Int J Med Inform. 2019;127:102-108. doi:10.1016/j.ijmedinf.2019.04.004
- Calibrate Dx: A Resource To Improve Diagnostic Decisions. Accessed September 4, 2024. https://www.ahrq.gov/diagnostic-safety/tools/calibrate-dx.html
- Poluch M, Feingold-Link J, Ankam N, et al. I Don't Have a Diagnosis for You: Preparing Medical Students to Communicate Diagnostic Uncertainty in the Emergency Department. MedEdPORTAL. 2022;18:11218. doi:10.15766/mep_2374-8265.11218
- Advancing safety with closed-loop communication of test results. Published online November 13, 2019. Accessed December 31, 2023. https://psnet.ahrq.gov/issue/advancing-safety-closed-loop-communication-test-results
- Lacson R, Prevedello LM, Andriole KP, et al. Four-year impact of an alert notification system on closed-loop communication of critical test results. AJR Am J Roentgenol. 2014;203(5):933-938. doi:10.2214/AJR.14.13064
- Yale SC, Cohen SS, Kliegman RM, Bordini BJ. A pause in pediatrics: implementation of a pediatric diagnostic time-out. Diagnosis (Berl). 2022;9(3):348-351. doi:10.1515/dx-2022-0010
- Rowin EJ, Lucier D, Pauker SG, Kumar S, Chen J, Salem DN. Does error and adverse event reporting by physicians and nurses differ? Jt Comm J Qual Patient Saf. 2008;34(9):537-545. doi:10.1016/s1553-7250(08)34068-9
- Murphy DR, Meyer AN, Sittig DF, Meeks DW, Thomas EJ, Singh H. Application of electronic trigger tools to identify targets for improving diagnostic safety. BMJ Qual Saf. 2019;28(2):151-159. doi:10.1136/bmjqs-2018-008086
- Singh H, Bradford A, Goeschel C. Operational measurement of diagnostic safety: state of the science. Diagnosis. 2021;8(1):51-65. doi:10.1515/dx-2020-0045
- Gupta A, Snyder A, Kachalia A, Flanders S, Saint S, Chopra V. Malpractice claims related to diagnostic errors in the hospital. BMJ Qual Saf. 2017;27(1):bmjqs-2017-006774. doi:10.1136/bmjqs-2017-006774
- Cifra CL, Jones KL, Ascenzi JA, et al. Diagnostic Errors in a PICU: Insights From the Morbidity and Mortality Conference. Pediatr Crit Care Med. 2015;16(5):468-476. doi:10.1097/PCC.0000000000000398
- Shojania KG, Burton EC, McDonald KM, Goldman L. Changes in rates of autopsy-detected diagnostic errors over time: a systematic review. JAMA. 2003;289(21):2849-2856. doi:10.1001/jama.289.21.2849
- Meeks DW, Meyer AND, Rose B, Walker YN, Singh H. Exploring new avenues to assess the sharp end of patient safety: an analysis of nationally aggregated peer review data. BMJ Qual Saf. 2014;23(12):1023-1030. doi:10.1136/bmjqs-2014-003239
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, America C on the LHCS in, Medicine I of. A Continuously Learning Health Care System. In: Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. National Academies Press (US); 2013. Accessed April 25, 2024. https://www.ncbi.nlm.nih.gov/books/NBK207218/
- Connor DM, Durning SJ, Rencic JJ. Clinical Reasoning as a Core Competency. Acad Med. 2020;95(8):1166-1171. doi:10.1097/ACM.0000000000003027
- Pauker SG, Kassirer JP. The threshold approach to clinical decision making. N Engl J Med. 1980;302(20):1109-1117. doi:10.1056/NEJM198005153022003
- Harris A, Pineles L, Baghdadiv JD, et al. Clinician Testing and Treatment Thresholds for Management of Urinary Tract Infection. Open Forum Infect Dis. 2023;10(9):ofad455. doi:10.1093/ofid/ofad455
- Testing Wisely. March 18, 2020. Accessed March 18, 2020. https://www.testingwisely.com/
- Essex C. Ulysses syndrome. BMJ. 2005;330(7502):1268.
- Ganguli I, Simpkin AL, Lupo C, et al. Cascades of Care After Incidental Findings in a US National Survey of Physicians. JAMA Netw Open. 2019;2(10):e1913325. doi:10.1001/jamanetworkopen.2019.13325
- Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-780. doi:10.1097/00001888-200308000-00003
- Richards JB, Hayes MM, Schwartzstein RM. Teaching Clinical Reasoning and Critical Thinking: From Cognitive Theory to Practical Application. Chest. 2020;158(4):1617-1628. doi:10.1016/j.chest.2020.05.525
- Saber Tehrani AS, Lee H, Mathews SC, et al. 25-Year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680. doi:10.1136/bmjqs-2012-001550
- Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499. doi:10.1001/archinte.165.13.1493
- Giardina TD, Woodard LD, Singh H. Advancing Diagnostic Equity Through Clinician Engagement, Community Partnerships, and Connected Care. J Gen Intern Med. 2023;38(5):1293-1295. doi:10.1007/s11606-022-07966-8
- Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med. 1999;340(8):618-626. doi:10.1056/NEJM199902253400806
- Lucas FL, Siewers AE, DeLorenzo MA, Wennberg DE. Differences in cardiac stress testing by sex and race among Medicare beneficiaries. Am Heart J. 2007;154(3):502-509. doi:10.1016/j.ahj.2007.04.044
- Kim C, Kabbani S, Dube WC, et al. Health Equity and Antibiotic Prescribing in the United States: A Systematic Scoping Review. Open Forum Infect Dis. 2023;10(9):ofad440. doi:10.1093/ofid/ofad440
- Kornblith AE, Fahimi J, Kanzaria HK, Wang RC. Predictors for under-prescribing antibiotics in children with respiratory infections requiring antibiotics. Am J Emerg Med. 2018;36(2):218-225. doi:10.1016/j.ajem.2017.07.081
- Gil LA, Asti L, Beyene TJ, Cooper JN, Minneci PC, Besner GE. Inequities in the Diagnosis of Pediatric Appendicitis in Tertiary Children's Hospitals and the Consequences of Delayed Diagnosis. J Surg Res. 2023;292:158-166. doi:10.1016/j.jss.2023.07.049
- Yedjou CG, Sims JN, Miele L, et al. Health and Racial Disparity in Breast Cancer. Adv Exp Med Biol. 2019;1152:31-49. doi:10.1007/978-3-030-20301-6_3
- Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Racial and ethnic disparities in access to liver transplantation. Liver Transpl. 2010;16(9):1033-1040. doi:10.1002/lt.22108
- Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA. 2019;322(2):113-114. doi:10.1001/jama.2019.5774
- SAFER Guides | HealthIT.gov. Accessed July 29, 2024. https://www.healthit.gov/topic/safety/safer-guides
- Agency for Healthcare Research and Quality. Improving Your Laboratory Testing Process. Accessed July 29, 2024. https://www.ahrq.gov/hai/tools/ambulatory-care/lab-testing-toolkit.html
- Reporting Clinical Test Results | AMA-Code. Accessed July 29, 2024. https://code-medical-ethics.ama-assn.org/ethics-opinions/reporting-clinical-test-results
- Bradford A, Lubin I, Cornish N, Morgan D, Singh H. Diagnostic Stewardship as a Model To Improve the Quality and Safety of Diagnosis. Agency for Healthcare Research and Quality (US); 2024. Accessed September 4, 2024. https://www.ahrq.gov/diagnostic-safety/resources/issue-briefs/dxsafety-dx-stewardship.html
- Singh H, Upadhyay DK, Torretti D. Developing Health Care Organizations That Pursue Learning and Exploration of Diagnostic Excellence: An Action Plan. Acad Med. 2020;95(8):1172-1178. doi:10.1097/ACM.0000000000003062
- Improving Education—A Key to Better Diagnostic Outcomes | Agency for Healthcare Research and Quality. Accessed July 29, 2024. https://www.ahrq.gov/diagnostic-safety/resources/issue-briefs/education-dx-outcomes.html
- Dahm MR, Cattanach W, Williams M, Basseal JM, Gleason K, Crock C. Communication of Diagnostic Uncertainty in Primary Care and Its Impact on Patient Experience: an Integrative Systematic Review. J Gen Intern Med. 2023;38(3):738-754. doi:10.1007/s11606-022-07768-y
- Hammer MM, Kapoor N, Desai SP, et al. Adoption of a Closed-Loop Communication Tool to Establish and Execute a Collaborative Follow-Up Plan for Incidental Pulmonary Nodules. AJR Am J Roentgenol. 2019;212(5):1077-1081. doi:10.2214/AJR.18.20692
- Giardina TD, Haskell H, Menon S, et al. Learning From Patients' Experiences Related To Diagnostic Errors Is Essential For Progress In Patient Safety. Health Aff (Millwood). 2018;37(11):1821-1827. doi:10.1377/hlthaff.2018.0698
- Fowler FJ, Epstein A, Weingart SN, et al. Adverse events during hospitalization: results of a patient survey. Jt Comm J Qual Patient Saf. 2008;34(10):583-590. doi:10.1016/s1553-7250(08)34073-2
- Walton MM, Harrison R, Kelly P, et al. Patients' reports of adverse events: a data linkage study of Australian adults aged 45 years and over. BMJ Qual Saf. 2017;26(9):743-750. doi:10.1136/bmjqs-2016-006339
- Bhise V, Sittig DF, Vaghani V, Wei L, Baldwin J, Singh H. An electronic trigger based on care escalation to identify preventable adverse events in hospitalised patients. BMJ Qual Saf. 2018;27(3):241-246. doi:10.1136/bmjqs-2017-006975
- Murphy DR, Meyer AND, Vaghani V, et al. Application of Electronic Algorithms to Improve Diagnostic Evaluation for Bladder Cancer. Appl Clin Inform. 2017;8(1):279-290. doi:10.4338/ACI-2016-10-RA-0176
- Singh H, Giardina TD, Forjuoh SN, et al. Electronic health record-based surveillance of diagnostic errors in primary care. BMJ Qual Saf. 2012;21(2):93-100. doi:10.1136/bmjqs-2011-000304



