Key points
- Different laboratory tests are available to diagnose leishmaniasis.
- Some of the diagnostic methods are only available in reference laboratories.
- In the United States, CDC provides reference diagnostic services for leishmaniasis.
Background
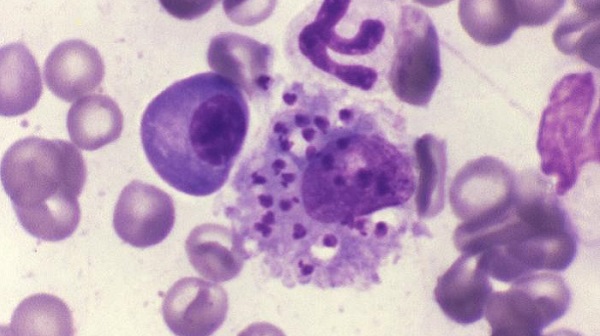
Various laboratory methods can be used to detect the parasite and to identify the Leishmania species. Some of the methods are available only in reference laboratories.
Determining a screening approach
Leishmaniasis is diagnosed by detecting Leishmania parasites (or DNA) in tissue specimens, such as from skin lesions (for CL) (see instructions), or from bone marrow (for VL). This can be done via light-microscopic examination of stained slides, molecular methods, and specialized culture techniques.
Identification of the Leishmania species also can be important, particularly if more than one species is found where the patient lived or traveled and if they can have different clinical and prognostic implications. The species can be identified by various approaches, such as molecular methods and biochemical techniques (isoenzyme analysis of cultured parasites).
For visceral leishmaniasis, serologic testing can provide supportive evidence for the diagnosis. The performance of various serologic assays may vary by geographic region and by host factors (e.g., the sensitivity of serologic testing generally is lower in patients coinfected with HIV, particularly if the HIV infection was acquired before the Leishmania infection). Most serologic assays do not reliably distinguish between active and quiescent infection.
Although leishmanin skin-test preparation is used in some endemic countries, this has not been approved for use in the United States.
Note: For VL, although the diagnostic sensitivity typically is higher for splenic aspirates than for specimens from other organs/tissues, splenic aspiration can be associated with life-threatening hemorrhage, even if conducted under radiologic guidance. Bone marrow aspiration is much safer. Examples of other potential sources of specimens for diagnostic testing include liver, lymph node, and whole blood or buffy coat. Among HIV-infected patients, the parasite also may be found in atypical sites (such as the gastrointestinal tract).
Resources
In the United States, CDC provides reference diagnostic services for leishmaniasis. CDC accepts both unpreserved and formalin-fixed specimens for testing, however, submission of specimens requires pre-approval. For more details and instructions in this regard, please see the CDC Test Directory.
