What to know
- Exposure to hepatitis viruses has long been recognized as an occupational risk for health care personnel (HCP).
- HCPs might be exposed to blood or other body fluids, by injury from a used needle, or from a splash of blood or body fluids into the eye or mouth while caring for a patient.
- This guidance provides CDC recommendations for laboratory testing and follow-up of HCPs who have potentially been exposed to hepatitis C virus (HCV) through an exposure to blood or other infectious body fluid.

About the recommendations
The 2020 CDC guidance, based on expert opinion, reflects current understanding of the viral dynamics of early HCV infection, as well as recent guidance from AASLD and IDSA, which recommends treatment of acute HCV infection.
Recommendation details
This guidance includes recommendations for a testing algorithm and clinical management for HCPs with potential occupational exposure to HCV.
Test the source patient
Testing guidance for source patients is described in consideration of the increasing incidence of acute HCV infection. HCPs can use the algorithm and recommendations to guide procedures for postexposure testing and clinical management of HCPs potentially exposed to HCV.
Test the source patient as soon as possible (preferably within 48 hours) after exposure. A source patient refers to any person receiving health care services whose blood or other potentially infectious material is the source of an HCP's exposure: See algorithm below.
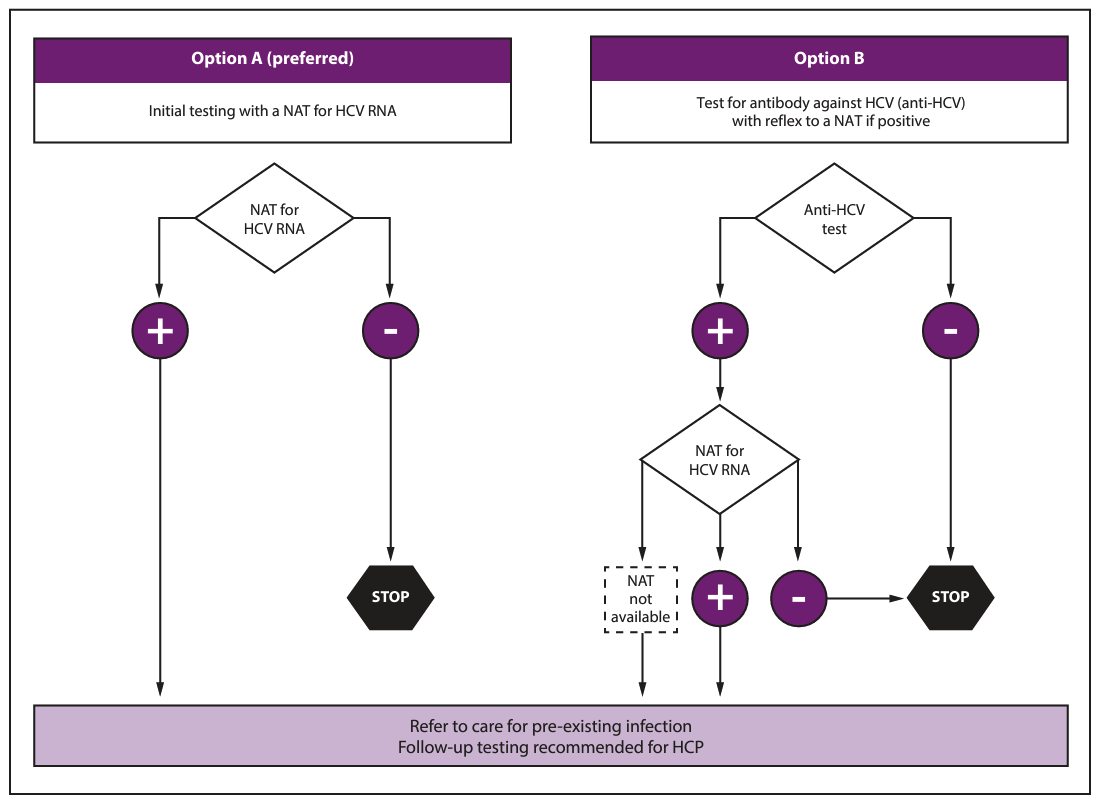
There are two options for testing the source patient. Option one is preferred, especially if the source patient is known or suspected to be at increased risk for HCV.
- Option one: Nucleic Acid Test (NAT) for HCV RNA.
- Option two: Hepatitis C antibody test (anti-HCV) with reflex to NAT if positive.
Source patient results
If the source patient is positive for HCV RNA, refer the patient for further care and evaluation for treatment as indicated in the American Association for the Study of Liver Diseases and Infectious Diseases Society of America (AASLD-IDSA) guidelines.
Follow-up testing of HCP is recommended if the source patient is:
- HCV RNA positive, OR
- Anti-HCV positive with HCV RNA status unknown, OR
- Cannot be tested.
Test the HCP
Simultaneous with source-patient testing, test the HCP as soon as possible (preferably within 48 hours) after exposure. See algorithm below:
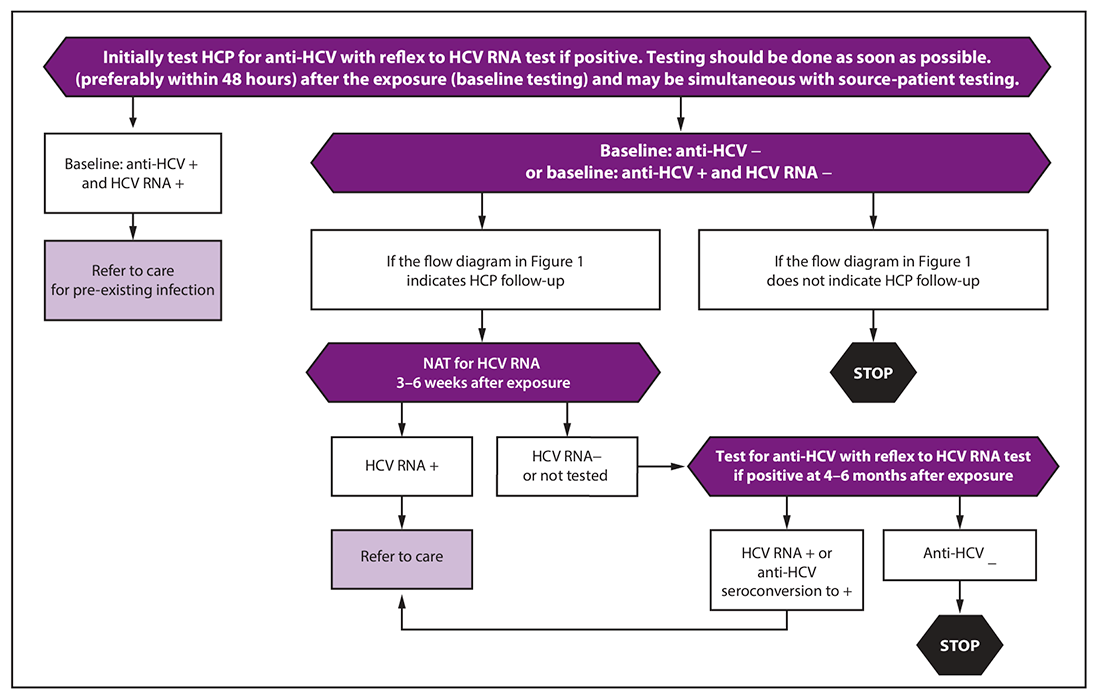
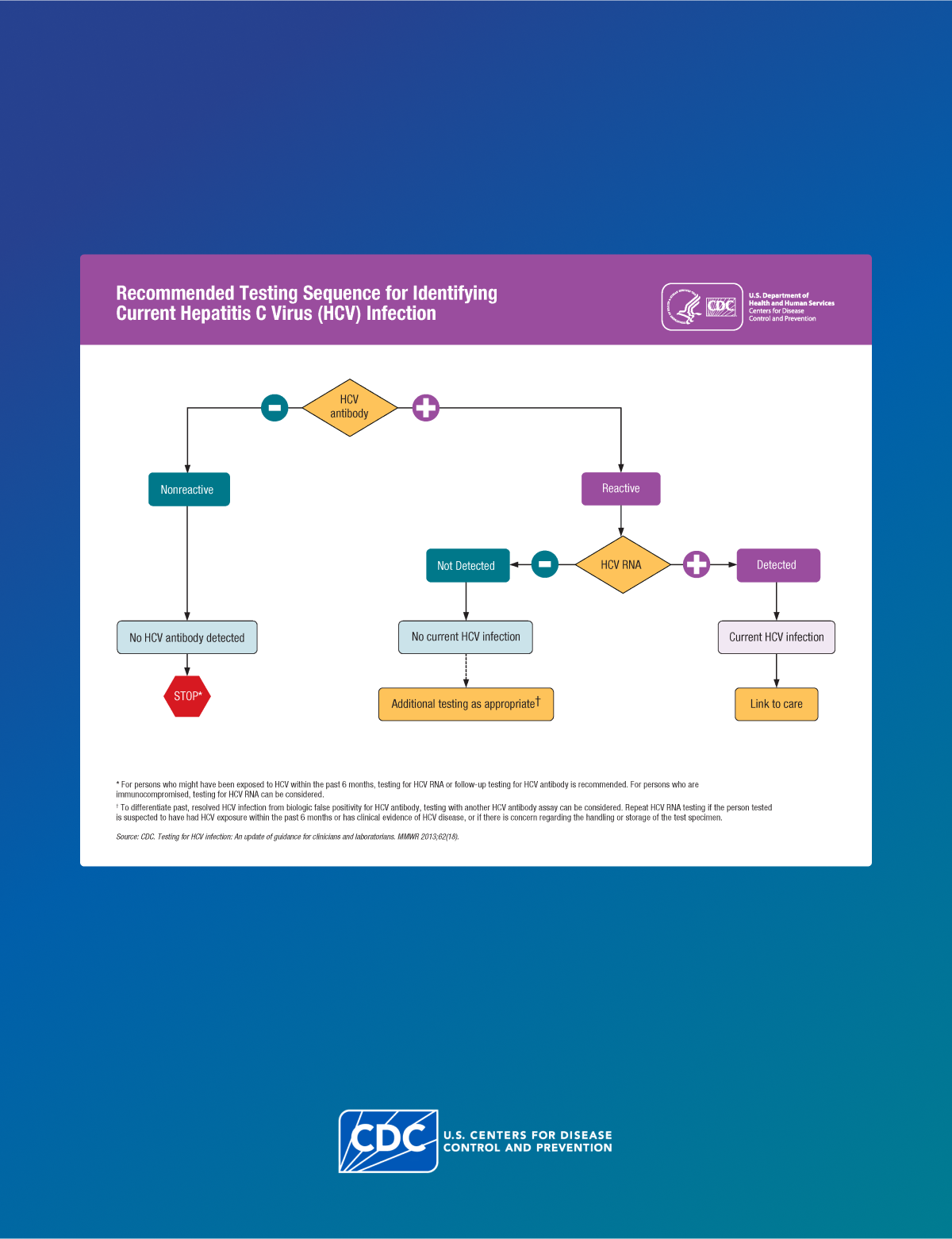
At baseline, test the HCP with an HCV antibody test with reflex to NAT for HCV RNA if positive.
Follow-up testing of HCP is recommended if the source patient is:
- HCV RNA positive, OR
- Anti-HCV positive with HCV RNA status unknown, OR
- Cannot be tested.
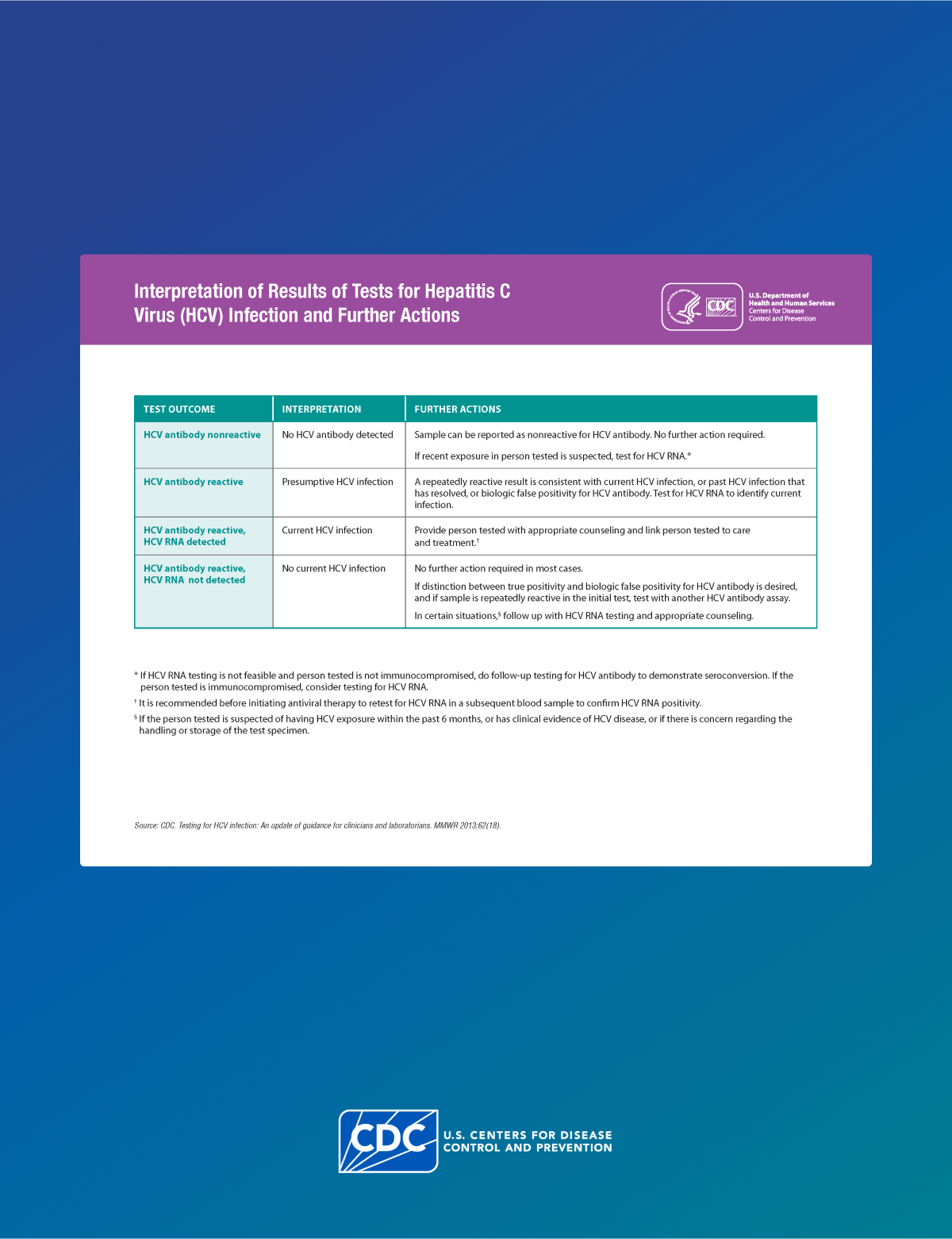
Follow-up testing and management of the HCP
If follow-up testing is recommended based on the source patient status, conduct a test 3–6 weeks after exposure with a NAT for HCV RNA.
If the HCP is HCV RNA negative at 3–6 weeks:
- Test one more time at 4–6 months after exposure for HCV antibody, with reflex to RNA if positive. (If the HCP was already HCV antibody positive but RNA negative at baseline, instead test for HCV RNA.)
- If the HCP remains anti-HCV negative at 4–6 months, no follow up is needed in most circumstances. However, if the HCP is immunocompromised or has liver disease, testing for HCV RNA can be considered.
If at any point the HCP has detectable HCV RNA or tests positive for anti-HCV after a previous negative test (seroconversion):
- Refer for further care and evaluation for treatment as indicated in AASLD-IDSA guidelines.
Additional considerations
- Note that if an HCV RNA test is positive but the RNA level is less than the lower limit of quantitation of the assay, the result may be reported as
- If there are concerns about results being compromised because of storage and handling errors or other issues that might affect specimen integrity: Repeat Tests.
- If the exposed HCP develops illness with symptoms indicative of acute HCV infection at any point: Test for HCV RNA.
Coinciding testing schedules
Human immunodeficiency virus (HIV)
Testing performed at 6 weeks postexposure has the advantage of coinciding with HIV postexposure testing schedules, if recommended.
Hepatitis B virus (HBV)
Testing performed at 6 months postexposure has the advantage of coinciding with HBV postexposure testing schedules, if recommended.
HCV postexposure prophylaxis (PEP)
CDC does not recommend HCV PEP with direct-acting antiviral (DAA) therapy for all potential exposures. The effectiveness and duration of treatment that would be required have not been established.
The risk for transmission of HCV from percutaneous exposures (0.2%) and mucocutaneous exposures (0%) is low. Further, routine PEP use for all occupational percutaneous exposures would treat approximately 1,000 HCV-exposed individuals for every two who might become infected.