Key points
- Hepatitis B virus (HBV) infection in a pregnant patient poses a serious risk to an infant at birth.
- HBV infection in infants can lead to long-term serious health effects if left untreated.
- Perinatal transmission of HBV infection is preventable.
- Vaccination is the best way to prevent HBV infection.

Overview
When a pregnant patient has HBV infection, it can pose a serious risk to their infant at birth.
Infants with HBV infection
Testing pregnant patients for hepatitis B and providing pregnant patients and infants with appropriate treatment and vaccination is the best way to prevent perinatal HBV infection.
Causes
The most common cause of perinatal HBV infection is when a pregnant patient with HBV infection gives birth and the infant does not receive postexposure immunoprophylaxis (hepatitis B birth dose and hepatitis B immune globulin [HBIG]) within 12 hours of birth1.
An infant can also develop HBV infection through contact with blood or other bodily fluids from a person with HBV infection.
Types
HBV infection can occur either with or without symptoms. HBV infection can be identified in pregnant patients and infants with a confirmed positive hepatitis B surface antigen (HBsAg) test result.
Acute hepatitis B infection that does not resolve progresses to chronic, or long-term, HBV infection. The risk for chronic HBV infection varies according to the age at infection and is greatest among young children. Approximately 90% of infants with HBV infection and 30% of children between ages 1–5 years with HBV infection will develop a chronic HBV infection. By contrast, approximately 95% of adults with HBV infection recover completely and do not become chronically infected.
Infection rates and trends
In 2021, an estimated 17,827 infants were born to HBsAg-positive people in the United States, representing nearly 0.5% of all births. Births to US-born and non-US-born people comprised 79% and 20% of all births, respectively, and 46% and 53% of estimated births to HBsAg-positive people, respectively.2
CDC collects, analyzes, and reports outcome data on infants born to people with HBV infection. This intervention is called the Perinatal Hepatitis B Prevention Program (PHBPP).3
Learn more about PHBPP outcome data.
Clinical features
Infants and children younger than 5 are typically asymptomatic.
If symptoms develop, they usually appear about 90 days after exposure (range: 60–150 days).
When present, signs and symptoms of HBV infection can include:
- Abdominal pain, nausea, and/or vomiting
- Dark urine or clay-colored stools
- Fatigue
- Fever
- Jaundice
- Joint pain
- Loss of appetite
Learn more about signs and symptoms of HBV infection.
Prevention
CDC's Advisory Committee on Immunization Practices (ACIP) recommends that all infants receive hepatitis B vaccine (HepB) at birth, regardless of the HBV infection status of the birthing parent1.
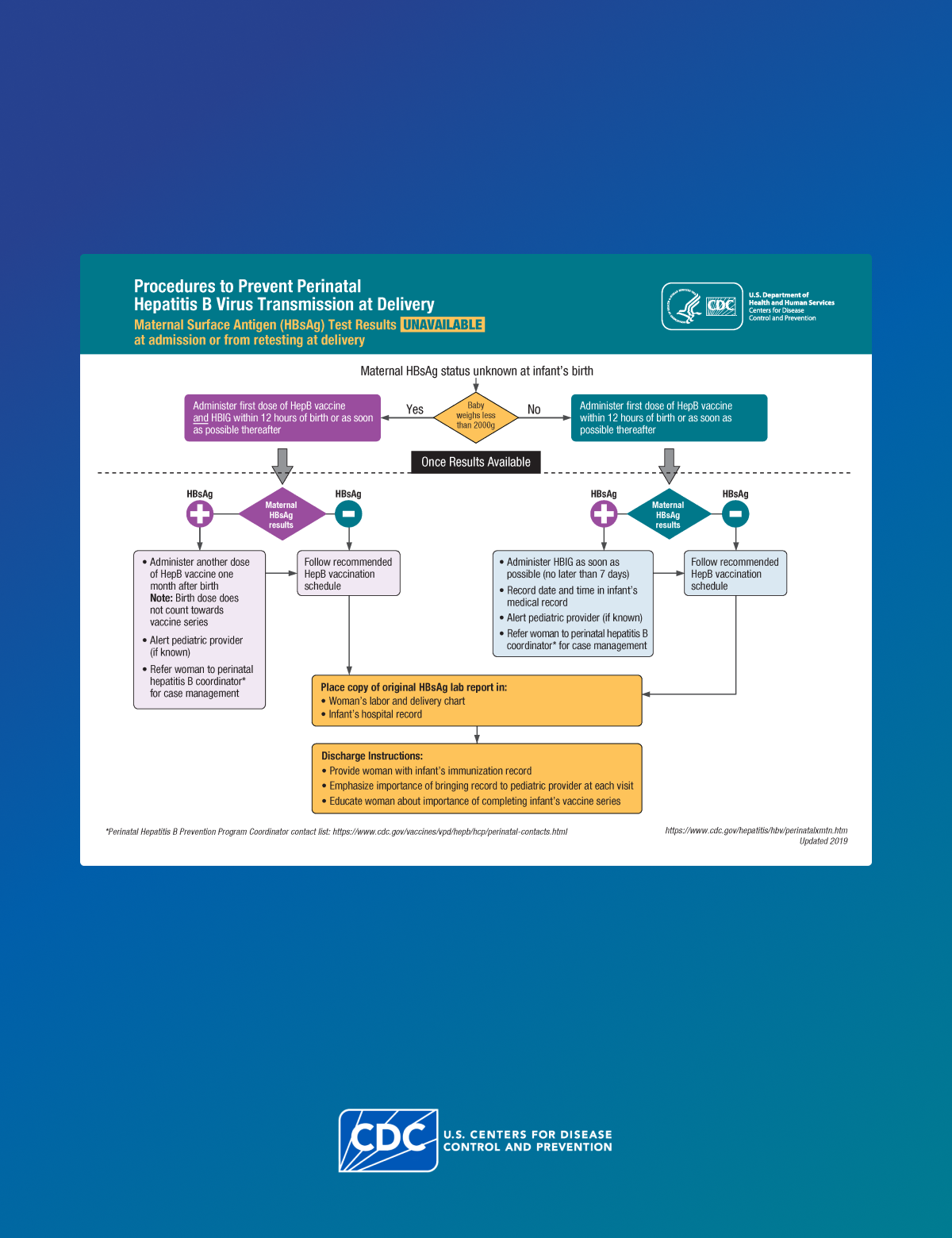
Infants born to HBsAg-positive people require HepB vaccine and HBIG within 12 hours of birth to protect them from infection. However, because errors or delays in testing, reporting, and documenting perinatal HBsAg status can and do occur, administering the first dose of HepB vaccine soon after birth to all infants acts as a safety net, reducing the risk for perinatal transmission when the HBsAg status of the parent is either unknown or incorrectly documented at delivery. Also, initiating the HepB vaccine series at birth has been shown to increase a child's likelihood of completing the vaccine series on schedule.
To learn more about HepB vaccine in infants, see Hepatitis B Perinatal Vaccine Administration.
Testing, screening, and diagnosis
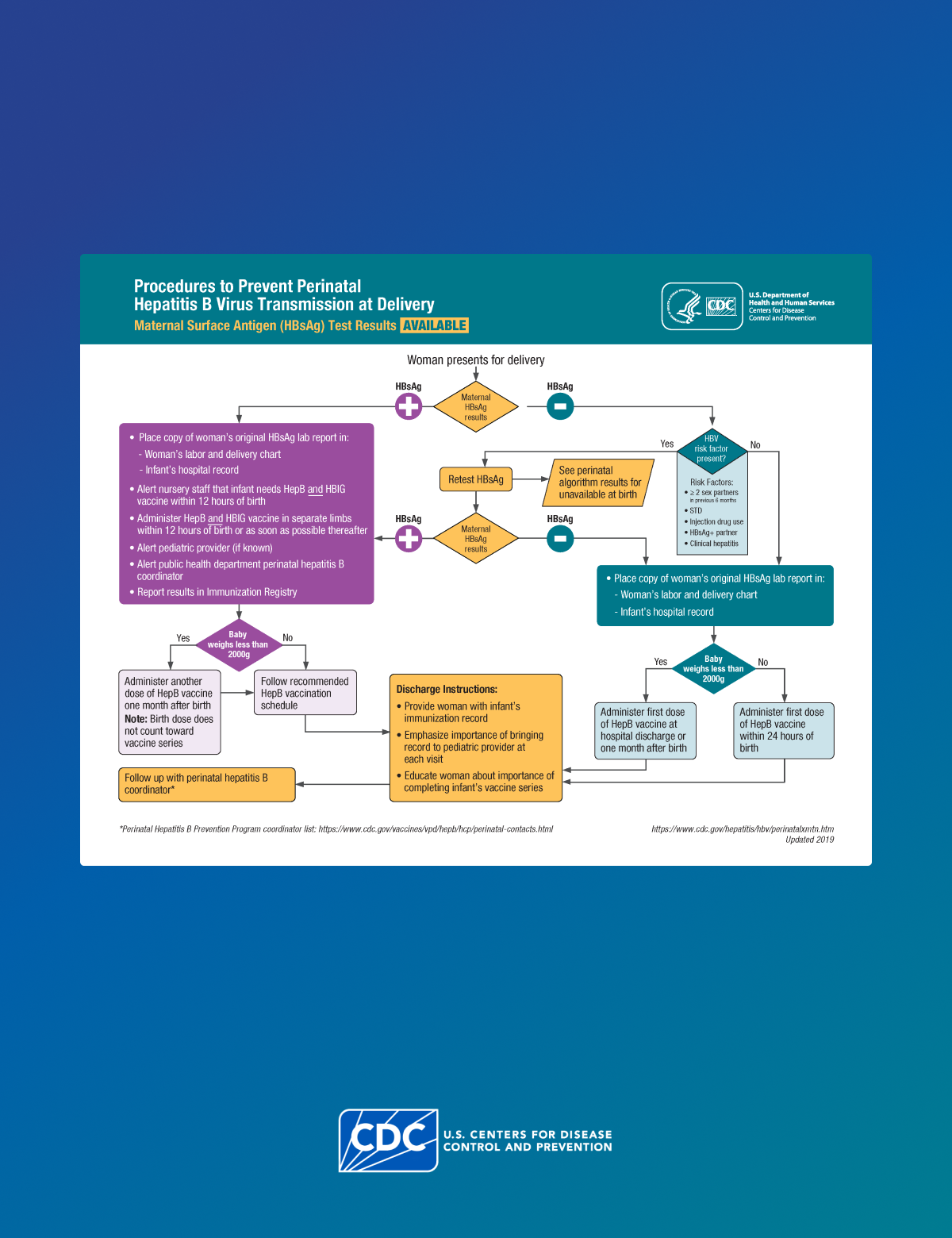
Clinicians should screen all pregnant patients for HBsAg during each pregnancy, preferably in the first trimester, regardless of vaccination status, chronic disease, or testing history. A confirmed positive HBsAg test result indicates current HBV infection.
Clinicians should test all infants born to an HBsAg-positive person that have completed the HepB vaccine series. Clinicians should test the infants for HBsAg and antibody to hepatitis B surface antigen (anti-HBs) at 9–12 months of age, or 1–2 months after vaccine series completion if the series is delayed.
To learn more about perinatal screening and testing, see perinatal testing guidelines.
Treatment and recovery
Once a clinician has confirmed test results, they can make treatment and management decisions.
Case management of pregnant patients with HBV infection
- All pregnant patients who are HBsAg-positive should be tested for HBV DNA to guide the use of maternal antiviral therapy during pregnancy for the prevention of perinatal HBV transmission.
- The American Association for the Study of Liver Diseases (AASLD) suggests maternal antiviral therapy when the maternal HBV DNA is greater than 200,000 IU/mL.
- All pregnant patients who are HBsAg-positive should be referred to their jurisdiction's PHBPP for case management to ensure that their infants receive timely postexposure immunoprophylaxis and follow-up. A copy of the original laboratory report indicating the pregnant patient's HBsAg-positive status should be provided to the hospital or birthing facility where the delivery is planned and to the health care provider (HCP) who will care for the newborn infant.
All infants born to people who are HBsAg-positive should receive HepB vaccine and HBIG within 12 hours of birth, administered at different injection sites (e.g., separate limbs). Only single-antigen HepB vaccine should be used for the birth dose.
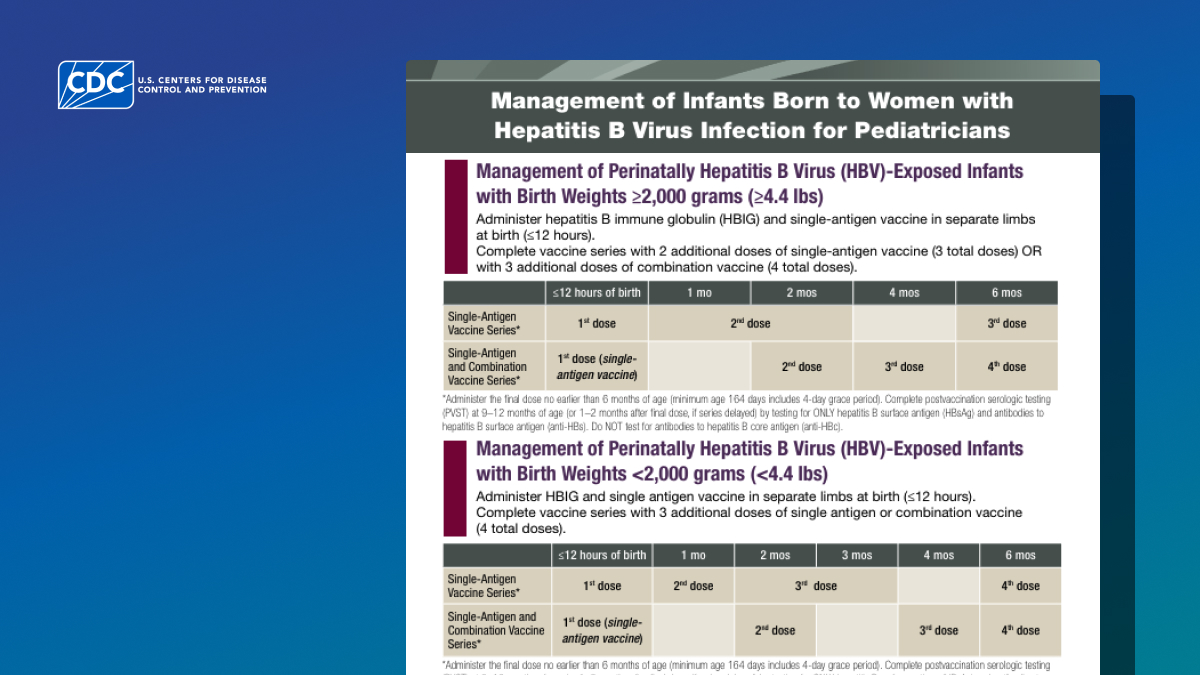
Management of perinatally HBV-exposed infants with birth weights ≥2,000 grams (≥4.4 lbs)
| ≤12 hours of birth | 1 mo | 2 mos | 4 mos | 6 mos | |
|---|---|---|---|---|---|
| Single-antigen vaccine series* | 1st dose | 2nd dose | 3rd dose | ||
| Single-antigen and combination vaccine series* | 1st dose (single-antigen vaccine) | 2nd dose | 3rd dose | 4th dose | |
Management of perinatally HBV-exposed infants with birth weights <2,000 grams (<4.4 lbs)
| ≤12 hours of birth | 1 mo | 2 mos | 3 mos | 4 mos | 6 mos | |
|---|---|---|---|---|---|---|
| Single-antigen vaccine series* | 1st dose | 2nd dose | 3rd dose | 4th dose | ||
| Single-antigen and combination vaccine series* | 1st dose (single-antigen vaccine) | 2nd dose | 3rd dose | 4th dose | ||
* Administer the final dose no earlier than 6 months of age (minimum age of 164 days includes 4-day grace period). Complete PVST at 9–12 months of age (or 1–2 months after final dose if series is delayed) by testing for ONLY HBsAg and anti-HBs. Do NOT test for anti-HBc.
The HepB vaccine series should be completed according to the recommended schedule above for infants born to an HBsAg-positive parent. The final dose in the series should not be administered before the age of 24 weeks (164 days).
Other important things to keep in mind:
- After completion of the vaccine series, post-vaccination serologic testing (PVST) for HBsAg and anti-HBs should be performed at age 9–12 months.
- Testing should not be performed before age 9 months to avoid detection of passive anti-HBs from HBIG administered at birth and to maximize the likelihood of detecting late HBV infection.
- Antibody to hepatitis B core antigen (anti-HBc) testing of infants is not recommended because passively acquired maternal anti-HBc might be detected in infants born to a person who is HBsAg-positive up to age 24 months.
For more information on management of infants, see Tip Sheet — Hepatitis B Screening, Testing, and Management of Pregnant Women for Obstetricians.
Patient counseling
Clinicians should provide all pregnant patients who test HBsAg-positive with:
- Information about the potential use of antiviral therapy for HBV infection.
- Information outlining the importance of timely postexposure immunoprophylaxis for their infant (HepB vaccine and HBIG within 12 hours of birth).
- Information on completing the vaccine series and PVST for their infant.
Long-term effects
HBV infection in a pregnant patient poses a serious risk to an infant at birth. Without timely postexposure immunoprophylaxis, approximately 90% of infants born to HBsAg-positive people in the US will acquire chronic HBV infection, approximately one-fourth of whom will eventually die from chronic liver disease.
Case definition
CDC, in collaboration with the Council of State and Territorial Epidemiologists, has developed case definitions to provide uniform clinical and laboratory-testing criteria of identifying and reporting nationally notifiable infections diseases.
To learn more about classification of perinatal hepatitis B cases, visit the: National Notifiable Diseases Surveillance System (NNDSS).
Public health efforts and research
HBV infection in a pregnant patient poses a serious risk to an infant at birth. Without timely postexposure immunoprophylaxis, approximately 90% of infants born to HBsAg-positive people in the US will acquire chronic HBV infection, approximately one-fourth of whom will eventually die from chronic liver disease.
Since 1990, CDC has funded public health jurisdictions (regions managed by state or local public health agencies) to identify pregnant patients with HBV infection and case manage their infants to prevent parent-to-child transmission of HBV infection. The national PHBPP aims to ensure infants born to people with HBV infection receive timely postexposure immunoprophylaxis, complete a full series of HepB vaccine, and receive PVST after a complete series to confirm immunity, identify infants needing revaccination, and identify infants who are infected. PHBPP-funded jurisdictions follow these infants for up to 24 months to monitor completion of these recommended interventions.3
Learn more about PHBPP outcome data.
Resources
For clinicians seeking more information, CDC has several resources available concerning perinatal HBV.
ACIP Presentation Slides: September 18, 2025
CDC resources
Downloadable algorithms
- Tip Sheet — Hepatitis B Screening, Testing, and Management of Pregnant Women for Obstetricians: This is a one-page hepatitis B resource for obstetricians managing pregnant patients.
- Tip Sheet — Management of Infants Born to People with HBV Infection for Pediatricians: This is a two-page tip sheet for pediatricians managing perinatally HBV-exposed infants.
American Society of Reproductive Medicine (ASRM) resources
Patient resources
When a Pregnant Woman Has Hepatitis B: These one-page resource covers the preventative measures a parent with hepatitis B can take to protect their baby.
- When a Pregnant Woman Has Hepatitis B (English)
- When a Pregnant Woman Has Hepatitis B (Spanish)
- When a Pregnant Woman Has Hepatitis B (French)
- When a Pregnant Woman Has Hepatitis B (Russian)
- When a Pregnant Woman Has Hepatitis B (Burmese)
- When a Pregnant Woman Has Hepatitis B (Chinese)
- When a Pregnant Woman Has Hepatitis B (Hmong)
- When a Pregnant Woman Has Hepatitis B (Khmer)
- When a Pregnant Woman Has Hepatitis B (Korean)
- When a Pregnant Woman Has Hepatitis B (Laotian)
- When a Pregnant Woman Has Hepatitis B (Tagalog)
- When a Pregnant Woman Has Hepatitis B (Vietnamese)
Hepatitis B and Your Baby: This one-page resource covers the benefits of Hep B vaccine for parents and their infants.
- Schillie S, Vellozzi C, Reingold A, et al. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2018;67(No. RR-1):1–31.
- National Center for Health Statistics. (2021) Expected Births [dataset].
- Koneru A, Fenlon N, Schillie S, et al. National Perinatal Hepatitis B Prevention Program: 2009–2017. Pediatrics March 2021;147(3)e20201823.