Key points
- CDC published updated recommendations among adults for hepatitis B screening and testing in 2023 that are complementary to the 2022 Advisory Committee on Immunization Practices (ACIP) vaccine recommendations for hepatitis B.
- The recommendations consider a simpler and less stigmatizing implementation strategy than previous risk-based hepatitis B virus (HBV) screening recommendations among adults.

Why it’s important
More than half of people with hepatitis B are unaware of their infection status, and approximately 50%–70% of people with acute hepatitis B are asymptomatic1. Without testing, people with hepatitis B virus (HBV) infection can unknowingly transmit the virus to others.
Chronic HBV infection can lead to substantial morbidity and mortality but is detectable before the development of severe liver disease using reliable and inexpensive screening tests. Routine monitoring and treatment for chronic HBV infection can reduce morbidity and mortality, supporting the importance of early detection of HBV infection.
In addition, although not quantifiable, management of chronic infection through prevention efforts can prevent further transmission to others.
Read more on the rationale for the new recommendations.
Who to test and screen
Adults and infants
Screen all adults aged 18 and older for hepatitis B at least once in their lifetime using a triple panel test. To ensure increased access to testing, anyone who requests HBV testing should receive it regardless of disclosure of risk. Many people might be reluctant to disclose stigmatizing risks.
Test all infants born to HBsAg-positive people for HBsAg and antibody to hepatitis B surface antigen (anti-HBs) seromarkers.
See the process flow diagram below to help guide HBC screening and testing decisions.
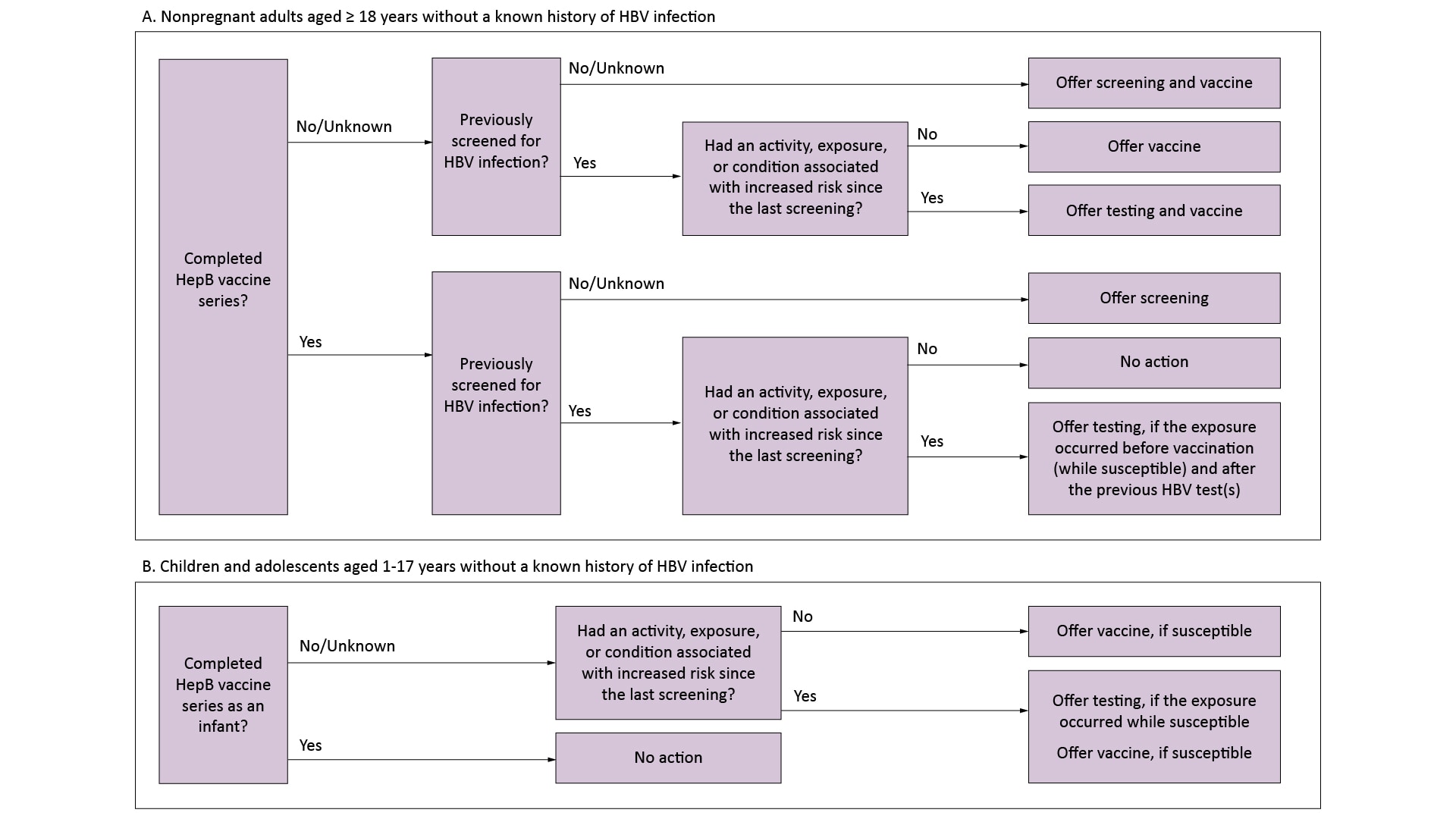
Pregnant Women
CDC recommends HBV screening for HBsAg for all pregnant women during each pregnancy, preferably in the first trimester, regardless of vaccination status or history of testing. Pregnant women with a history of appropriately timed triple panel screening without subsequent risk for exposure to HBV (no new HBV exposures since triple panel screening) only need HBsAg screening.
People at increased risk
CDC recommends testing susceptible people periodically, regardless of age, with ongoing risk for exposures while risk for exposures persists. This includes:
- People with a history of sexually transmitted infections or multiple sex partners.
- People with history of past or current HCV infection.
- People incarcerated or formerly incarcerated in a jail, prison, or other detention setting.
- Infants born to HBsAg-positive people.
- People born in regions with HBV infection prevalence of 2% or more.
- US-born people not vaccinated as infants whose parents were born in geographic regions with HBsAg prevalence of 8% or more.
- People who inject drugs or have a history of injection drug use.
- People with human immunodeficiency virus (HIV) infection.
- Men who have sex with men.
- Household contacts or former household contacts of people with known HBV infection.
- People who have shared needles with or engaged in sexual contact with people with known HBV infection.
- People on maintenance dialysis, including in-center or home hemodialysis and peritoneal dialysis.
- People with elevated liver enzymes.
Susceptible people include those who have never been infected with HBV and either did not complete a hepatitis B vaccine (HepB) series per ACIP recommendations or who are known to be vaccine nonresponders.
The table below provides a quick view of recommendations for screening and testing among adults and all persons at increased risk.
| Population | Screening and testing recommendation |
|---|---|
| Adults with no known risk factors for hepatitis B | If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel) |
| People with risk factors, regardless of age* | If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel) • Unless less than aged 18 years and completed a vaccine series as an infant If previously screened, but still unvaccinated, offer testing to people who have ongoing risk for exposure |
| People with additional risk factors, such as: • Residents and staff of facilities for people with developmental disabilities • Health care and public safety personnel with reasonably anticipated risk for exposure to blood or blood-contaminated body fluids • People with diabetes at the discretion of the treating clinician International travelers to countries with high or intermediate levels of endemic hepatitis B virus infection |
If never previously screened, test for HBsAg, anti-HBs, and total anti-HBc (triple panel) • Unless less than aged 18 years and completed a vaccine series as an infant |
*For additional considerations for patients on dialysis, see Footnote A.A
Recommended tests
CDC now* recommends use of the triple panel test, which includes testing for:
- HBsAg
- Anti-HBs
- Total antibody to hepatitis B core antigen (total anti-HBc)
Any periodic follow-up testing can use tests as appropriate based on the results of the triple panel.
*Prior guidance recommended a single test of HBsAg.
How to interpret test results
Different serologic markers or combinations of markers are used to identify different phases of HBV infection. They determine whether a patient has acute or chronic HBV infection, is immune to HBV as a result of prior infection or vaccination, or is susceptible to infection. Markers include:
- HBsAg: HBsAg is a protein on the surface of HBV that can be detected in high levels in serum during acute or chronic HBV infection. The presence of HBsAg indicates that the person is infectious, except when it might be transiently positive within 30 days after a dose of HepB vaccine. The body normally produces antibodies to HBsAg as part of the normal immune response to infection. HBsAg is the antigen used to make HepB vaccine.
- Anti-HBs: The presence of anti-HBs is generally interpreted as indicating recovery and immunity from HBV infection. Anti-HBs also develops in a person who has been successfully vaccinated against hepatitis B. Among vaccine responders who complete a vaccine series, anti-HBs levels can decline over time; however, the majority remain immune and will mount a response when exposed to HBV.
- Anti-HBc: Anti-HBc appears at the onset of symptoms in acute hepatitis B, is a measure of both immunoglobulin M (IgM) and immunoglobulin G (IgG), and persists for life. The presence of total anti-HBc indicates previous or ongoing infection with HBV in an undefined time frame. People who have immunity to hepatitis B from a vaccine do not develop anti-HBc.
- IgM anti-HBc: IgM anti-HBc positivity indicates recent infection with HBV (within less than 6 months). Its presence indicates acute infection. IgM anti-HBc should be ordered only when acute HBV infection is a concern.
| Test outcome | Interpretation | Action |
|---|---|---|
| HBsAg — Positive Total anti-HBc — Positive IgM anti-HBc — Positive* Anti-HBs — Negative |
Acute infection | Link to hepatitis B care |
| HBsAg — Positive Total anti-HBc — Positive IgM anti-HBc — Negative Anti-HBs — Negative |
Chronic infection | Link to hepatitis B care |
| HBsAg — Negative Total anti-HBc — Positive Anti-HBs — Positive |
Resolved infection | Counsel about HBV infection reactivation risk |
| HBsAg — Negative Total anti-HBc — Negative Anti-HBs — Positive† |
Immune from receipt of prior vaccination (if documented complete series) | If not vaccinated, then complete vaccine series |
| HBsAg — Negative Total anti-HBc — Positive Anti-HBs — Negative |
Only core antibody is positive. See possible interpretations and corresponding actions. | |
| Resolved infection where anti-HBs levels have waned | Counsel about HBV infection reactivation risk | |
| Occult infection | Link to hepatitis B care | |
| Passive transfer of anti-HBc to an infant born to an HBsAg-positive gestational parent | No action | |
| False positive, thus patient is susceptible | Offer HepB vaccine per ACIP | |
| A mutant HBsAg strain that is not detectable by laboratory assay | Link to hepatitis B care | |
|
HBsAg — Negative Total anti-HBc — Negative Anti-HBs — Negative‡ |
Susceptible, never infected (if no documentation of HepB vaccine series completion) | Offer HepB vaccine per ACIP recommendations |
How to diagnose hepatitis B
The presence of the total anti-HBc antigen is needed to diagnose a patient with a hepatitis B infection. The results of the HBsAg, anti-HBs, and IgM anti-HBc tests indicate a patient’s type of hepatitis B and if they have developed immunity.
Learn more about viral hepatitis serology.
What to do next
CDC recommends that people who are diagnosed with hepatitis B be provided with:
- Medical evaluation (by either a primary care clinician or specialist for chronic liver diseases) including treatment and monitoring.
- Supportive care for their symptoms as needed.
For more CDC information on recommendations for testing, management, and treatment of hepatitis B, see Clinical Care of Hepatitis B.
Reporting cases
The National Notifiable Diseases Surveillance System (NNDSS) lists acute, chronic, and perinatal hepatitis B as nationally notifiable conditions.
You should report cases of suspected health care-associated HBV infection to state and local public health authorities for prompt investigation and response.
When you report a case, you will need an event code corresponding to the hepatitis B condition. You can reclassify cases if needed, as long as the changes occur before surveillance data are finalized each year.
National event codes:
- Acute hepatitis B: 10100
- Perinatal hepatitis B: 10104
- Chronic hepatitis B: 10105
Resources
Scientific guidelines and recommendations
Other resources
- Kodani M, Schillie SF. Chapter 4: Hepatitis B. In: Roush S, Baldy LM, Kirkconnell Hall MA, eds. Manual for the Surveillance of Vaccine-Preventable Diseases, 2020.
