At a glance
When it comes to diagnosing drug-resistant TB (DR-TB), a quick, accurate diagnosis is critical. The global Xpert TB Proficiency Testing (XTPT) program was created to assess the quality of TB and DR-TB diagnostic services across CDC-supported countries and ensure a timely diagnosis. The program has helped to assess and improve the quality and capacity of TB and DR-TB testing services globally, helping to find and accurately diagnose cases for improved TB control.
Proficiency testing for DR-TB diagnosis

CDC International Laboratory Branch
Drug-resistant tuberculosis (DR-TB) poses a significant threat to global health security as it has become the leading cause of death associated with antimicrobial resistance. DR-TB, now found in every country of the world, is deadlier, costlier, and harder to treat than diseases that respond to antibiotic treatment, making it a severe public health crisis and putting millions of lives at risk.
When it comes to diagnosing DR-TB, timing is critical. Once the laboratories receive a sample, the clock starts ticking to identify the bacterium that causes the disease, Mycobacterium tuberculosis, and quickly and accurately determine if the sample responds to antibiotic treatment. Diagnosing DR-TB in this way requires specific skills, materials, and equipment that are not easily acquired.
Proficiency testing programs
Laboratories participate in proficiency testing (PT) programs to test their laboratory skills and assess whether the materials and machines needed are available and working properly to meet the recommended timelines. Through these programs, labs receive and test a selection of samples. The labs test the samples and report their findings to the PT program who then scores results for all enrolled labs, providing each lab with a performance report that indicates if the correct results were achieved in time. These performance reports help labs that were not 100% successful identify and address gaps in their services to ensure that patients receive the most accurate, reliable, and timely results for clinical action and disease control.
Revolutionizing TB testing
In 2010, a revolutionary test, Xpert MTB/RIF, replaced the cumbersome century-old testing procedure for TB—helping to improve case findings by 40% while also testing for DR-TB. GeneXpert multi-disease testing instruments reduced the time to complete tests from 2-9 weeks to just two hours. With this new technology, the number of DR-TB diagnoses doubled.
The same year, Xpert MTB/RIF became the first diagnostic test to be endorsed as a World Health Organization-recommended diagnostic for TB. This endorsement revolutionized TB diagnostic services for doctors, patients, and clinics—especially in high-burden TB countries. To meet the new demand, Xpert TB tests underwent a rapid and large-scale roll-out in high-burden TB countries. However, PT programs lagged. Many countries had limited access to PT services because biosafe, transportable testing materials were either unavailable or too expensive, so they were unable to verify that their new testing sites provided accurate results for patient care. By 2012, less than 42% of the global Xpert TB testing sites were enrolled in a PT program, and only 24% of enrolled sites were in the African region.
Intervention
With support from the U.S. President's Emergency Plan for AIDS Relief (PEPFAR), CDC adapted the Dried Tube Specimen (DTS) PT sample technology that the agency had previously developed for rapid HIV testing. Innovating DTS for use in Xpert TB PT proved successful as the testing materials could be made safely, accurately, and precisely, and the materials would remain stable over a range of cold and hot temperatures. CDC also produced these DTS for TB using equipment and supplies that would already be available in functional TB culture labs in high-burden settings. This ensured that laboratories in resource-limited settings could produce their own DTS in the future.
Recognizing its potential for success and the widespread need and demand for Xpert TB PT services, CDC designed a global Xpert TB Proficiency Testing (XTPT) program to support quality assessment of TB and DR-TB diagnostic services across CDC-supported countries.
In 2013, CDC successfully piloted the XTPT program in ten sites in South Africa. Over the next year, the pilot program expanded to 100 sites across seven countries, followed by increases in country and site enrollment every year. By the end of 2024, the program had successfully scaled to over 1,460 testing sites across 27 countries.
In 2016, CDC increased sustainability measures by developing and implementing training programs for laboratory professionals to produce DTS in-country and establish national PT programs, starting with Vietnam, India, and Uganda. After establishing the first XTPT program in India, CDC provided support to scale-up their program, enrolling over 2,000 TB Xpert testing sites and expanding their PT services to include other new TB and DR-TB tests recommended for use by the World Health Organization. Vietnam and Uganda also established and successfully scaled-up their national programs, reaching 100% coverage of their Xpert TB testing networks while successfully adapting PT services for new tests.
Building on the success of the XTPT program, CDC collaborated with the TB Supranational Reference Laboratory in Uganda (SRL-Uganda) and the National TB Reference Laboratory (NTRL) in Vietnam. By 2019, with CDC's support, these independent programs began serving as regional DTS production and XTPT program training centers to increase their reach to nearby countries.
Impact
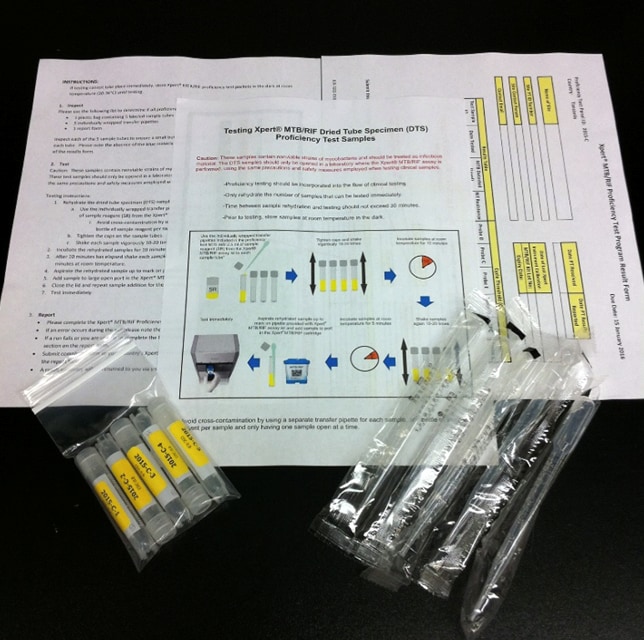
CDC International Laboratory Branch
Each regional center has achieved tremendous success. CDC and SRL-Uganda have trained Botswana, Kenya, Malawi, and Nigeria on PT program development, while SRL-Uganda trained an additional seven countries (Benin, Ethiopia, Mozambique, South Sudan, Tanzania, Zambia, and Zimbabwe) during the COVID-19 pandemic. The Vietnam NTRL also began providing regional PT support to Laos, Myanmar, and the Philippines, and later added support for Bangladesh and Papua New Guinea. They also began training the Philippines to establish their own program which provides support for more than 900 laboratories.
- Dr. Nguyen Van Hung, director of the Vietnam NTRL
To support the expansion of PT program establishment and facilitate sharing of successes, challenges, and lessons learned, CDC has also collaborated with the African Field Epidemiology Network (AFENET) to establish the world's first proficiency testing Community of Practice (COOP) in the African region. Virtual and video-enhanced COOP calls were held biweekly for countries and programs to cross-share their successes, best practices, and solutions to challenges and participate in technical learning sessions; all supported by University of New Mexico's Project ECHO technology.
In just 10 years, XTPT has transformed from a new, innovative CDC global public health service to a multi-region, sustainably funded, locally led PT operation in 17 countries around the globe. The program has helped to assess and improve the quality and capacity of TB and DR-TB testing services globally, helping to find and accurately diagnose cases for improved TB control.
In the decade since the program was first piloted, CDC has shipped 19,075 PT panels and made up over 95,375 dried tube specimens for DR-TB testing sites. In 2022 alone, the programs participating in the African COOP enrolled over 2,200 Xpert TB testing sites and distributed over 4,400 PT panels. COOP results indicate that 93 percent of participating sites achieved a satisfactory score, showcasing a high level of accuracy in reporting across the diagnostic testing sites and identifying gaps where improvements can be made.
Building capacity for new PT programs
The World Health Organization continues to endorse new molecular diagnostic tests that will need corresponding PT programs so laboratories can assess the accuracy of their services. To meet this need, CDC is assessing and optimizing DTS sample preparation for new testing methods and working on transferring these technological advances to partner countries. CDC is also working with partners to develop a new virtual training course to help meet the increasing demand for program support. The course will allow countries to develop DTS and design their independent PT programs in real time, supporting countries to establish and customize these new services for their unique settings.
CDC continues to build support for testing and quality assurance programs in high-burden partner countries to ensure patients worldwide have access to accurate TB diagnostic test results. CDC's proficiency testing program is an innovative example of how one country cannot end TB alone. Through collaborative partnerships and thoughtful advances in scientific technology, we can support countries in utilizing limited resources to detect critical diseases more accurately and reliably.
