At a glance
Chapter 3 of The CDC Field Epidemiology Manual
Introduction
When a threat to the public's health occurs, epidemiologists are ready responders who investigate the problem so they can identify causes and risk factors, implement prevention and control measures, and communicate with everyone involved. Epidemiologic field investigations are a core function of epidemiology and perhaps the most obvious way information is transformed into action to ensure public health and safety (see Chapter 1). This chapter describes the step-by-step process required in performing an epidemiologic field investigation. The 10 steps covered here build on and further refine the steps that have been taught traditionally in the Centers for Disease Control and Prevention's (CDC) annual Epidemic Intelligence Service courses, in the three previous editions of this manual (the textbook Field Epidemiology), and in other CDC instructional programs. The 10 steps discussed here are similar to those found in other epidemiology instructional publications12345. Lists, take-home points, and examples are provided to clarify key aspects and improve the practical utility of the discussion. This chapter describes a field investigation in the context of a public health response to a presumed acute infectious disease outbreak, although this approach also applies to other scenarios and problems.
Background Considerations
An outbreak is defined as "the occurrence of more cases of disease than expected in a given area or among a specific group of people over a particular period of time"1. When there are clearly many more cases than usual that are distributed across a larger geographic area, the term epidemic can be used. An outbreak is a situation that usually needs a rapid public health response. Notification of a suspected outbreak can come from different sources, including astute clinicians, laboratory scientists, public health surveillance data, or the media.
After the decision is made to start an investigation, clearly defining the objective of the investigation is crucial. Field investigations of common outbreak scenarios have standard objectives and time-tested methods that can be implemented rapidly. For example, because transmission modes associated with foodborne and waterborne outbreaks are well-known (i.e., spread by contact with infected persons, animals, or contaminated food or water), epidemiologists have developed the National Hypothesis Generating Questionnaire6, a standardized questionnaire to help develop hypotheses and collect information from ill persons regarding demographics and specific exposures. In contrast, at the time of initial recognition, many outbreaks have no obvious or known cause, which challenges the epidemiologist to establish a clear objective early—albeit one that is broad and can be revised as the investigation evolves—and to generate hypotheses. (see How Case Definition and Outbreak Focus Change: Zika Virus Infection).
Finally, a certain urgency to field investigations and pressure to find an answer quickly will always exist. For example, rapid surveys or other study designs used in outbreak investigations might lack the level of statistical power or proof of causality that often are possible in prospectively planned research studies. Likewise, delays caused by waiting for all laboratory samples to be tested can delay determination of pathogens or modes of spread and, consequently, implementation of control measures. However, the goal is to be both timely and accurate. Because of these considerations, coordinating with all partners and establishing priorities early is key to a successful investigation.
Adapted from Reference7
The Investigation
Epidemiologists use a systematic multistep approach to field investigations. Although these steps are presented here in a numeric order, they might be conducted out of order or concurrently to meet the demands of the investigation. For example, in certain circumstances, implementing a control measure soon after notification and confirmation of an outbreak might be possible and even advisable. Often, Steps 2 (Confirm the Diagnosis) and 3 (Determine the Existence of an Outbreak) are performed at the same time. These two steps highlight the need for increased collaboration (or teamwork) early in the investigation among public health officials, laboratory personnel, clinicians, and other stakeholders.
Ten Steps of a Field Investigation8
Step 1. Prepare for Field Work
An important first step in any field investigation is addressing the operational aspects related to preparing for field work (see Chapter 2). This preparation includes ensuring that all persons involved agree on the purpose of the investigation and that the required official approvals for the field investigation have been received. A formal invitation for assistance must be received from an authorized official; for example, when a state requests assistance from CDC to conduct an investigation, the Governor or an appropriate public health officer like the state epidemiologist would be authorized to extend that invitation. In addition, roles and responsibilities of those involved in the investigation must be delineated. For most investigations, laboratory testing will play a crucial role; thus, discussions with laboratory colleagues about types of testing and specimens need to occur before the field investigation begins. Concerns related to the safety of the field team (e.g., whether personal protective equipment will be needed) also should be considered during this first step. Ensuring that this early preparation for the field investigation is completed will prevent misunderstandings and other problems later.
Step 2. Confirm the Diagnosis
Confirming or verifying the diagnosis ensures, to the extent possible, that you are addressing the problem that was reported initially and rules out misdiagnosis and potential laboratory error. For example, in a communicable disease outbreak, the real clustering of false infections—a consequence of misdiagnosis and laboratory error—can result in a pseudoepidemic. The term pseudoepidemic refers to a situation in which there is an observed increase in positive test results, or the incidence of disease related to something other than a true increase in disease. Diagnoses can be confirmed by implementing some or all of the following activities:
- Interviewing the affected persons;
- Clinical examination of the affected persons by health-care personnel when indicated and possible;
- Reviewing medical records and other pertinent clinical information (e.g., radiography and other imaging studies); and
- Confirming the results of laboratory testing; if the epidemiologist does not have the expertise to assess the adequacy, accuracy, or meaning of the laboratory findings, laboratory scientists and other personnel should be consulted.
Although laboratory data might be the best and only link between a putative cause and case, not every case requires laboratory confirmation before further action can be taken. A related step to confirming diagnoses is the need to obtain specimens (e.g., microbiologic strains already isolated) before they have been discarded so that they are available for further analysis if new questions arise later in an investigation.
Step 3. Determine the Existence of an Outbreak
Determining the existence of an outbreak is sometimes a difficult step that should be completed before committing program resources to a full-scale investigation. This step also is necessary to rule out spurious problems (e.g., pseudoepidemics or reporting increases caused by surveillance artifacts). As noted previously, pseudoepidemics might result from real clustering of false infections (e.g., inadvertent contaminants of laboratory specimens) or artifactual clustering of real infections (e.g., increases in the number of reported cases because of changes in surveillance procedures introduced by the health department or implemented by a healthcare delivery system).9 Problems potentially associated with pseudoepidemics include risks related to unnecessary or inappropriate treatment and unnecessary diagnostic procedures.
To confirm the existence of an outbreak, the field investigation team must first compare the number of cases during the suspected outbreak period with the number of cases that would be expected during a non-outbreak timeframe by
- Establishing a comparison timeframe in the suspected epidemic setting by considering, for example, whether it should be the period (e.g., hours, days, weeks, or months) immediately preceding the current problem or the corresponding period from the previous year;
- Taking into account potential problems or limitations in determining comparison timeframes (e.g., lack of data, varying or lack of case definitions, incomplete reporting, and other reasons for inefficient surveillance); and
- Calculating occurrence rates, when possible, between the period of the current problem and a comparator period.
For certain problems, an outbreak can be rapidly confirmed through use of existing surveillance data. For others, however, substantial time lags might occur before a judgment can be made about the existence of an outbreak.
Step 4. Identify and Count Cases
The aim of this step is to identify, or ascertain, as many cases as possible without including non-cases. As a practical matter, this entails casting a broad net through use of a classification scheme—the case definition (see the following discussion)—that maximizes sensitivity (i.e., correctly identifies persons who have cases of the condition [true-positives]) and optimizes specificity (i.e., does not include persons who do not have cases of the condition [false-positives] (See Simple Versus Complex Case Definitions).
The case definition is a statement consisting of three elements that together specify a person
- With a condition consisting of (a) a set of symptoms (e.g., myalgia or headache) or (b) signs (e.g., elevated temperature, maculopapular rash, or rales) or (c) laboratory findings (e.g., leukocytosis or positive blood culture); and
- With the condition occurring during a particular period, usually referred to as the epidemic period; and
- With the condition occurring after the person was in one or more specific settings (e.g., a hospital, school, place of work, or community or neighborhood, or among persons who participated in a gathering, such as a wedding or meeting).
Although the case definition might be broad at the onset of an epidemiologic field investigation, it is a flexible classification scheme that often is revised and narrowed as the investigation progresses.
To minimize the likelihood of ascertainment bias (i.e., a systematic distortion in measurement due to the way in which data are collected), cases ideally are sought and counted through systematic searches of a multiplicity of potential sources to identify the maximum number, or a representative sample, of cases. Examples of sources include
- Public health agency surveillance data;
- Medical system records from hospitals, laboratories, or ambulatory care settings;
- Institutional setting records (e.g., school and workplace attendance records); and
- Special surveys.
Information about identified cases (e.g., coded patient identifiers, age, sex, race/ethnicity, date of illness onset or diagnosis, symptoms, signs, laboratory findings, or other relevant data) should be systematically recorded in a spreadsheet or through other means (e.g., a line listing or similar epidemiologic database) for subsequent analysis and for use in conducting further investigative studies (e.g., hypothesis testing). All staff involved in data collection and maintenance should be trained to use the forms and questionnaires (whether these be on paper or electronic) and to store the forms to protect personal information while facilitating rapid data analysis.
Depending on the nature, scope, and extent of the outbreak, consideration should be given to the need for additional active case finding and surveillance once sufficient information has been collected to support prevention and control efforts. Specifically, ongoing or intensified surveillance can be paramount in subsequent efforts to evaluate the effectiveness of control measures for curbing and terminating the epidemic (see Step 9).
Simple Versus Complex Case Definitions
Example of a Simple Case Definition
The 2007 Zika virus (ZIKV) outbreak in Yap used the following case definition:
Case definition: A patient with suspected disease had acute onset of generalized macular or papular rash, arthritis or arthralgia, or nonpurulent conjunctivitis during the period from April 1 through July 31, 2007.
Case classification: We considered a patient to have confirmed Zika virus disease if Zika virus RNA was detected in the serum or if all the following findings were present: IgM antibody against Zika virus (detected by ELISA), Zika virus PRNT90 titer of at least 20, and a ratio of Zika virus PRNT90 titer to dengue virus PRNT90 titer of at least 4. A patient was classified as having probable Zika virus disease if IgM antibody against Zika virus was detected by ELISA, Zika virus PRNT90 titer was at least 20, the ratio of Zika virus PRNT90 titer to dengue virus PRNT90 titer was less than 4, and either no Zika virus RNA was detected by RT-PCR or the serum sample was inadequate for the performance of RT-PCR.
Example of a Complex Case Definition
Note how the case definition changed from 2007 as researchers learned more about ZIKV transmission.
Laboratory Criteria for Diagnosis
Recent ZIKV Infection
- Culture of ZIKV from blood, body fluid, or tissue; OR
- Detection of ZIKV antigen or viral ribonucleic acid (RNA) in serum, cerebrospinal fluid (CSF), placenta, umbilical cord, fetal tissue, or other specimen (e.g., amniotic fluid, urine, semen, saliva); OR
- Positive ZIKV immunoglobulin M (IgM) antibody test in serum or CSF with positive ZIKV neutralizing antibody titers and negative neutralizing antibody titers against dengue or other flaviviruses endemic to the region where exposure occurred.
Recent Flavivirus Infection, Possible ZIKV
- Positive ZIKV IgM antibody test of serum or CSF with positive neutralizing antibody titers against ZIKV and dengue virus or other flaviviruses endemic to the region where exposure occurred,
- Positive ZIKV IgM antibody test AND negative dengue virus IgM antibody test with no neutralizing antibody testing performed.
Epidemiologic Linkage
- Resides in or recent travel to an area with known ZIKV transmission; OR
- Sexual contact with a confirmed or probable case within the infection transmission risk window of ZIKV infection or person with recent travel to an area with known ZIKV transmission; OR
- Receipt of blood or blood products within 30 days of symptom onset; OR
- Organ or tissue transplant recipient within 30 days of symptom onset; OR
- Association in time and place with a confirmed or probable case; OR
- Likely vector exposure in an area with suitable seasonal and ecological conditions for potential local vector borne transmission.
See Also Subtype Case Definitions
- Zika virus disease, congenital
- Zika virus disease, noncongenital
- Zika virus infection, congenital
- Zika virus infection, noncongenital
Source12
Step 5. Tabulate and Orient the Data in Terms of Time, Place, and Person
This step involves translating and transforming data from the line listing into a basic epidemiologic description of the outbreak. This description characterizes the outbreak in terms of time, place, and person (referred to as descriptive epidemiology). Through systematic review of data in the line listing, key actions typically involve
- Drawing epidemic curves,
- Constructing spot maps or other special spatial projections, and
- Comparing groups of persons.
In addition, these key actions contribute to developing initial hypotheses for explaining the potential cause, source, and mode of spread of the outbreak's causative agent(s).
Time
Establishing the time of the outbreak or epidemic requires the following actions:
- Develop a chronologic framework by collecting information about and ordering key events identified during creation of the line listing or through other inquiry, including
- Time of onset of illness (symptoms, signs, or laboratory test positivity) among affected persons;
- Period of likely exposure to the causal agent(s) or risk factor(s);
- Time when treatments were administered or control measures were implemented; and
- Time of potentially related events or unusual exposures.
Chapter 6 includes examples of epidemic curves displaying the types of information that can be analyzed to aid in conducting a field investigation.
- Develop an epidemic curve by graphing the number of cases on the y-axis in relation to units of time (e.g., hours, days, months) on the x-axis—note that time intervals conventionally should be less than (i.e., one-fourth to one-third) the known or suspected incubation period.
- Use the epidemic curve configuration to make preliminary inferences about the modes of spread (e.g., person-to-person, common-source, or continuing point source) of a suspected causative agent.
- If the agent is known, use knowledge of the incubation period to look retrospectively at the period of likely exposure among affected persons.
- If the agent is unknown, but a common event or exposure period is likely, consider potential causal agents on the basis of the possible incubation period.
- When indicated, construct epidemic curves relative to specific sites (e.g., workplace settings, hospital units, classrooms, or neighborhoods) or groups identified by other potential risk characteristics.
Place
Use information collected for the line listing and through other inquiry to orient cases in relation to locations, including
- Place of residence,
- Place of occupation,
- Venues for recreational activity;
- Activity sites (e.g., rooms or units in which persons were hospitalized; rooms visited during a convention or meeting; or seating or activity locations on transportation conveyances, such as planes or cruise ships).
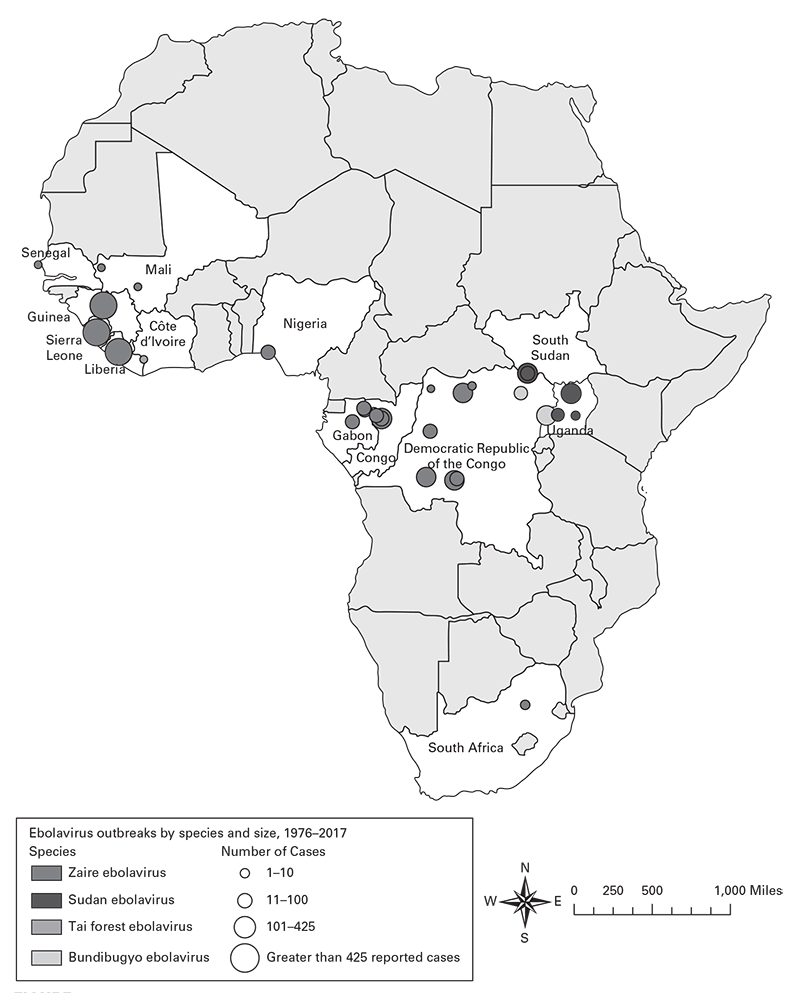
Using information about place, construct spot maps (Figures 3.1, 3.2, 3.3) or other visual methods to depict locations of cases at time of onset of illness or possible exposure to causal agents or factors, including
- Within buildings
- City blocks or neighborhoods, or
- Geographic or geopolitical areas (e.g., cities, counties, states, or regions).
Person
Use information collected for the line listing to describe cases in relation to such factors as
- Demographic characteristics (e.g., age, sex, and race/ethnicity), occupation, and diagnoses; and
- Features shared by affected persons.
When possible and where indicated, obtain denominator data (e.g., total cook-out attendees in a foodborne disease outbreak) to develop preliminary estimates of rates of illness in relation to demographic, exposure, and other characteristics.
Figure 3.113
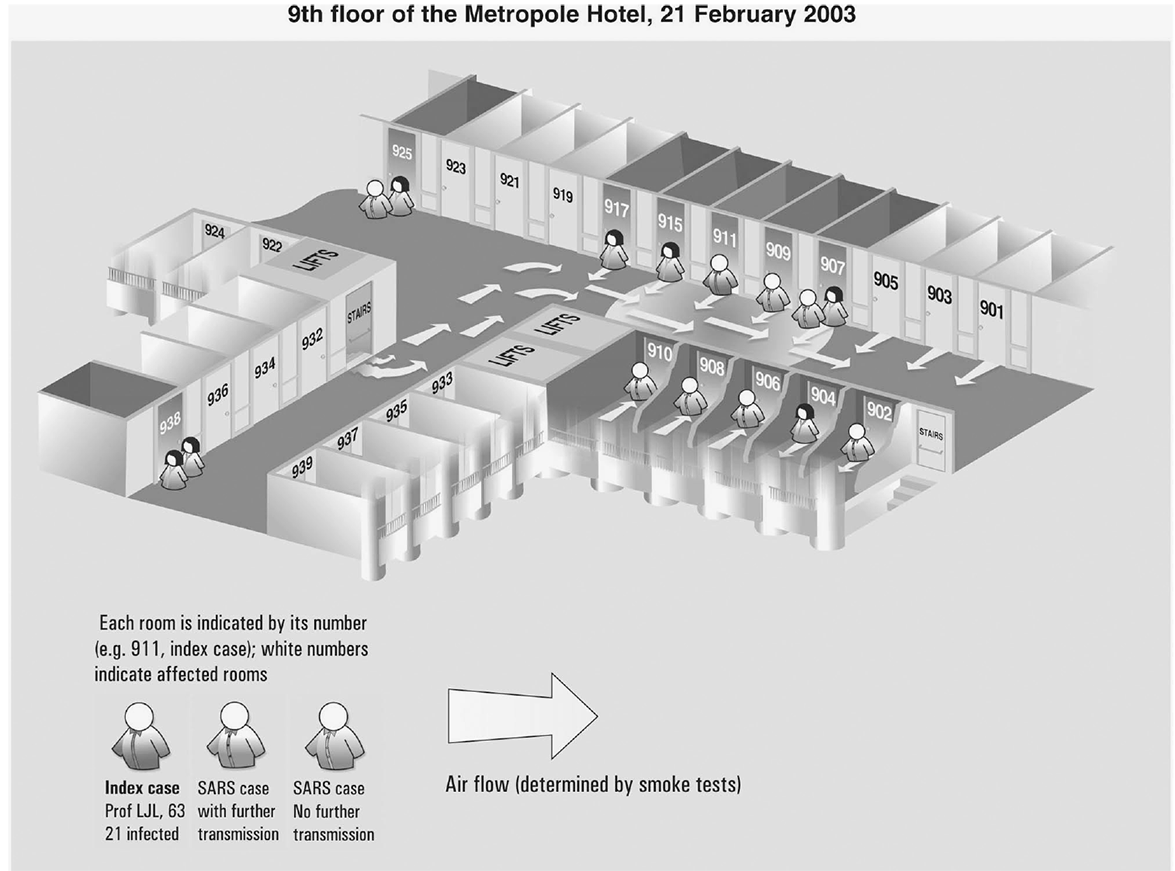
Figure 3.214
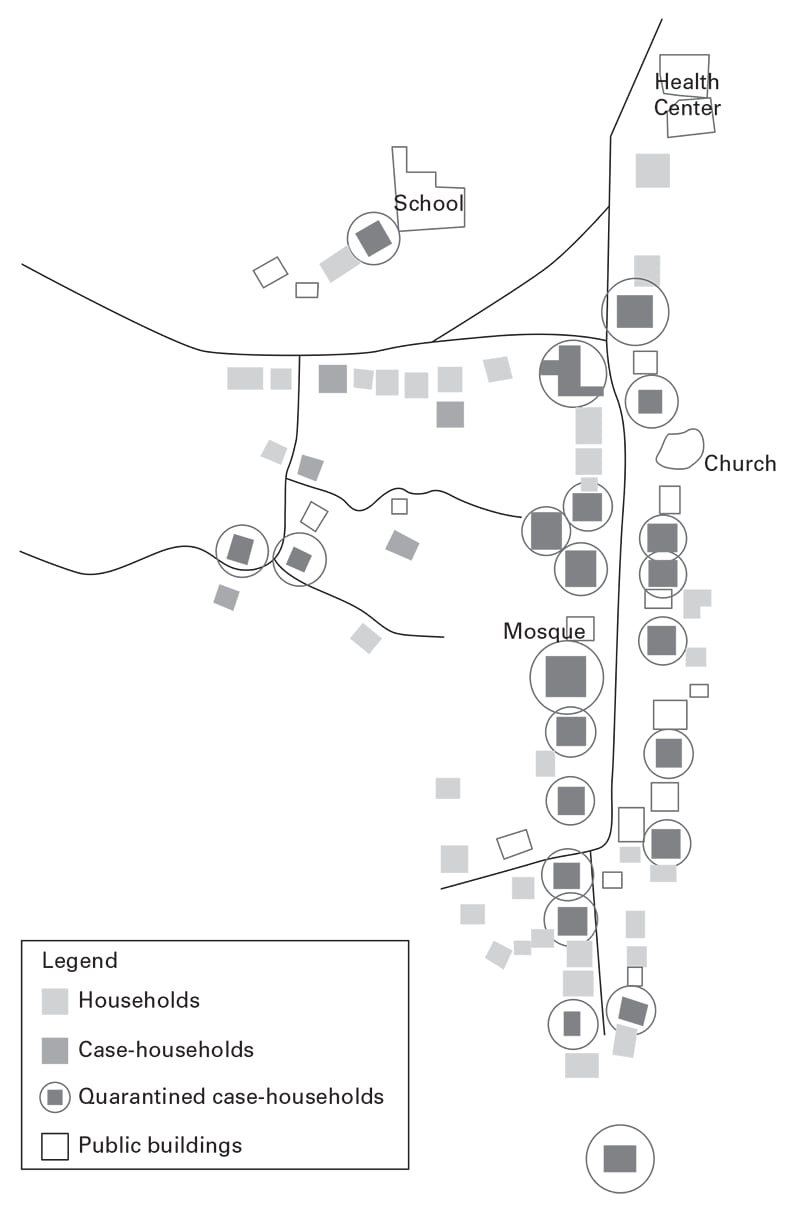
Figure 3.315
Step 6. Consider Whether Control Measures can be Implemented Now
Control measures include two categories of interventions:1 those that can be directed at the source(s) of most infectious and other disease-causing agents (e.g., treating infected persons and animals or isolating infected persons who are contagious) and2 those that can be directed at persons who are susceptible to such agents (administering postexposure prophylaxis, vaccinating in advance, or employing barrier techniques) (See Chapter 11 and Public Health Example: Controlling an Outbreak of Hepatitis A in a Child Day Care Setting). In concept, control measures are implemented only after the preceding and subsequent steps—including developing and testing hypotheses about the cause or mode of spread—have been implemented. In practice, however, decisions about control measures might be necessary at any step in the sequence, and preliminary control measures can be instituted on the basis of limited initial information and then modified as needed as the investigation proceeds. Control measures should be considered again after more systematic studies are complete.
Adapted from Reference16
Step 7. Develop and Test Hypotheses
Hypotheses about the disease-causing agent, source or reservoir of the agent, transmission mode, and risk factors for disease can be developed based on information from multiple sources including:
Expert subject-matter knowledge by field epidemiologists, laboratory colleagues, and others;
- Descriptive epidemiologic findings resulting from analysis of the line listing of identified affected persons;
- Information obtained from interviews of individuals or groups of affected persons by using structured questionnaires or open-ended questioning;
- Anecdotes, impressions, and ideas from affected persons or others in the affected area; and
- Consideration of outlier cases (i.e., cases with onset occurring at the beginning or end of the outbreak period).
In certain instances, descriptive epidemiologic findings alone, or results of cross-sectional survey data or other studies will be sufficient for developing hypotheses. Often, however, analytic epidemiologic methods—especially cohort or case– control studies—will be needed for identifying possible risk and other causative factors and for testing the strength of the association of the factors with the disease. The process of hypothesis testing, therefore, can entail multiple iterations of hypothesis generating and testing, serial studies, and collection, analysis, and management of considerable additional data. See Chapter 7 for description of how cohort and case– control studies can be used effectively in foodborne or waterborne disease outbreaks and other types of field investigations (see Public Health Example: Hypothesis Formulation).
Typically, statistically significant (e.g., small p value) findings of associations alone do not constitute an adequate body of evidence to support conclusions about the validity of hypotheses and to implement interventions to terminate an outbreak. Instead, all key information and investigative findings should be viewed as a whole in relation to such standards as the Bradford Hill tenets of causation17 (see Bradford Hill Criteria).
Step 8: Plan One or More Systematic Studies
At this stage of most epidemiologic field investigations, the purposes of systematic or other studies might include improving the quality of information underlying the investigation’s conclusions about the problem (e.g., improving the quality of numerators or denominators). Additional examples include refining the accuracy of the estimates of persons at risk and examining other germane concerns (e.g., expanding characterization of the causative agent and its epidemiology).
Adapted from Reference18
Step 9. Implement and Evaluate Control and Prevention Measures
The ultimate purpose of an epidemiologic field investigation is to implement scientifically rational and advisable control measures for preventing additional outbreak-associated morbidity or mortality. Control measures implemented in outbreaks will vary based on the causative agent; modes of spread; size and characteristics of the population at risk; setting; and other considerations, such as available resources, politics, and community concerns. Categories of control measures used for terminating outbreaks are described in this chapter in Step 6 and are further addressed in Chapter 11.
Evaluating the impact of control measures is essential. Therefore, evaluation efforts should be implemented concurrently with control measures to assess their effectiveness in attenuating and ultimately terminating the outbreak. If not yet in place, active surveillance should be initiated to monitor for new cases and for evidence of effect of the control measures and to guide decision-making about additional needs (e.g., further investigation, additional studies, or modifications to the control measures).
Regardless of the intervention, the ethical implications of any action must be considered. Because outbreak investigations typically involve collection of private, personally identifiable information from individual persons, and often from their families, coworkers, or other acquaintances, epidemiologists should be familiar with applicable local, state, and federal laws regarding privacy protections.
Bradford Hill Criteria17
- Strength of association.
- Consistency with other studies.
- Temporality (exposure precedes effect [illness]).
- Biologic plausibility.
- Biologic (dose-response) gradient.
Step 10: Communicate Findings
Field epidemiologists must be diligent and effective communicators throughout and after outbreak investigations. The information they provide helps keep the public and stakeholders accurately apprised during an outbreak, informs decisions about actions to halt the outbreak, and documents the investigation.
This step requires the following actions:
- Establish a communications plan at the onset of the investigation (see also Chapter 2).
- Identify and designate a spokesperson or a consistent point of contact who will serve as the primary communicator for the investigative team. This will optimize the team's efficiency by concentrating the communications role in one person who is accessible to the news media and others. This also minimizes the potential for confusion or misunderstanding by ensuring consistency in messaging throughout the investigation.
- Provide oral briefings and written communications, as might be indicated.
- Written reports can be customized for multiple purposes, including formally conveying recommendations, meeting institutional requirements for documentation, providing a record for future reference, and facilitating rapid dissemination of investigation findings to the requesting authority, stakeholders, scientific colleagues, and others.
- Before departing the field, the investigative team should provide a preliminary written report and oral briefing to the requesting authority and local stakeholders that documents all activities, communicates the findings, and conveys recommendations. A final, more detailed, report might be provided later, especially if additional analyses and studies are planned.
- Brief reports published rapidly in public health bulletins (e.g., Morbidity and Mortality Weekly Report) can help alert colleagues about the problem.
Conclusion
This chapter presents a 10-step approach to conducting an epidemiologic field investigation. Although no steps should be skipped, they might be conducted concurrently or out of order depending on the circumstances of the investigation. Although descriptive epidemiologic findings are sufficient for supporting initiation of public health action in certain investigations, more extensive inquiry, including analytic studies, often is required to provide a scientifically rational basis for interventions. Regardless of complexity, the list of steps that organize epidemiologic field investigations helps to ensure focus and thoroughness throughout the investigative response.
- CDC. Self-study course SS 1978. Principles of epidemiology in public health practice, third edition. An introduction to applied epidemiology and biostatistics. Lesson six: investigating an outbreak.
- Gertsmann BB. Outbreak investigation. In: Gertsman BB, ed. Epidemiology kept simple: an introduction to traditional and modern epidemiology. 2nd ed. Hoboken, NJ: Wiley-Liss, Inc.; 2003:351–64.
- Brownson RC. Outbreak and cluster investigations. In: Brownson RC, Petitti DB, eds. Applied epidemiology: theory to practice. New York: Oxford University Press; 1998:71–104.
- Reingold AL. Outbreak investigations—a perspective. Emerg Infect Dis. 1998;4:21–7.
- CDC. Multistate and nationwide foodborne outbreak investigations: a step-by-step guide. https://www.cdc.gov/foodborne-outbreaks/
- CDC. Foodborne disease outbreak investigation and surveillance tools: national hypothesis generating questionnaire. https://www.cdc.gov/foodborne-outbreaks/
- Imperato PJ. The convergence of a virus, mosquitoes, and human travel in globalizing the Zika epidemic. J Community Health. 2016;41:674–9.
- Gregg MB. Conducting a field investigation. In: Gregg MB, ed. Field epidemiology. 3rd ed. New York: Oxford University Press; 2008:81–96.
- Weinstein RA, Stamm WE. Pseudoepidemics in hospital. Lancet. 1977;310:862–4.
- CDC. Pneumocystis pneumonia—Los Angeles. MMWR. 1981;30:250–2.
- CDC. Task Force on Kaposi's Sarcoma and Opportunistic Infections. Epidemiologic aspects of the current outbreak of Kaposi's sarcoma and opportunistic infections. N Engl J Med. 1982;306:248–52.
- Duffy MR, Chen TH, Thane Hancock W, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536–2543; and Centers for Disease Control and Prevention. National Notifiable Disease Surveillance System (NNDSS). Zika virus disease and Zika virus infection, 2016 case definition. https://ndc.services.cdc.gov/conditions/zika-virus-disease-and-zika-virus-infection/
- World Health Organization. SARS: how a global epidemic was stopped. WHO Regional Officer for the Western Pacific Region. Manila, Philippines: World Health Organization; 2006. http://www.yncdc.cn/newsview.aspx?id=19185
- Gleason B, Foster S, Wilt G, et al. Geospatial analysis of household spread of Ebola virus in a quarantined village—Sierra Leone, 2014. Epidemiol Infect. 2017;145:2921–9.
- CDC. Ebola virus disease distribution map. https://www.cdc.gov/ebola/outbreaks/
- Goodman RA, Buehler JW, Koplan JP. The epidemiologic field investigation: science and judgment in public health practice. Am J Epidemiol. 1990;132:9–16.
- Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300.
- Cavallaro E, Date K, Medus C, et al. Salmonella Typhimurium infections associated with peanut products. N Engl J Med. 2011;365:601–10.
