Volume 29, Number 9—September 2023
Research
COVID-19 Epidemiology during Delta Variant Dominance Period in 45 High-Income Countries, 2020–2021
Abstract
The SARS-CoV-2 Delta variant, first identified in October 2020, quickly became the dominant variant worldwide. We used publicly available data to explore the relationship between illness and death (peak case rates, death rates, case-fatality rates) and selected predictors (percentage vaccinated, percentage of the population >65 years, population density, testing volume, index of mitigation policies) in 45 high-income countries during the Delta wave using rank-order correlation and ordinal regression. During the Delta-dominant period, most countries reported higher peak case rates (57%) and lower peak case-fatality rates (98%). Higher vaccination coverage was protective against peak case rates (odds ratio 0.95, 95% CI 0.91–0.99) and against peak death rates (odds ratio 0.96, 95% CI 0.91–0.99). Vaccination coverage was vital to preventing infection and death from COVID-19 during the Delta wave. As new variants emerge, public health authorities should encourage the uptake of COVID-19 vaccination and boosters.
The Delta variant of SARS-CoV-2, first identified in India in October 2020 (1), became the dominant variant in >130 countries worldwide during June–November 2021 (2). Global vaccination coverage during that time remained low; <50% of the world’s population had received >1 dose and <25% had completed a primary vaccination series (3). Despite the increased transmissibility of Delta compared with previous variants, countries experienced varying levels of illness and death (4).
With each new wave of COVID-19, governments’ responses varied depending on the understanding of SARS-CoV-2, variant characteristics, and societal factors such as healthcare system capacity, vaccination coverage, and the public’s willingness to follow public health mitigation measures. Policies ranged from flattening the curve (i.e., slowing down infection rates to alleviate pressure on healthcare systems) to zero-COVID policies that aimed to completely prevent infections in the community (5–7).
As the COVID-19 pandemic continues and new variants emerge (8), governments are working to identify and implement a combination of effective yet socially and economically acceptable measures. For example, previous works showed the effectiveness of nonpharmaceutical interventions (e.g., remote work, mask-wearing in indoor public spaces) in reducing case rates and death rates (9–11). In addition, 1 study showed that fewer deaths were reported in countries with earlier and more stringent mitigation policies, such as business closures, restrictions on public gatherings, and stay-at-home orders (12). Some countries employing zero-COVID policies experienced rising cases and deaths during the initial Omicron period and beyond (13). The benefits and social acceptability of prolonged lockdowns and mass testing policies to prevent new cases and deaths remain unclear (14).
We aimed to describe the epidemiology during the pre-Delta and Delta wave periods of the COVID-19 pandemic among high-income countries (HICs) and characterize the relationship between public health policies and epidemiologic burden. Specifically, we assessed the degree to which key measures of illness and death (case rate, death rate, and case-fatality rate) were correlated with vaccination coverage, testing volume, mitigation stringency, population density, and demographics.
Inclusion Criteria
We restricted our analysis to HICs because more accessible testing in those countries likely provides a more accurate assessment of the case and death burdens (15,16). We included HICs that reported >200 SARS-CoV-2 sequences during the full period of interest (April 1, 2020–November 30, 2021) and >20 Delta sequences in the first month of Delta dominance. The cutoff of 200 sequences was chosen to ensure adequate reflection of variant burden. In August 2021, the World Health Organization (WHO) recommended a minimum of 15 specimens per week (1); 200 sequences would provide >3 months’ worth of data. We chose 20 Delta sequences as a minimum to prevent the overrepresentation of a few sequences during weeks that had slower reporting. We defined Delta dominance as the time during which >50% of sequenced samples were Delta.
Data Sources
We obtained data for this study from 4 publicly available sources. First, we used World Bank country income classifications for 2020 (17) to select HICs. This classification is updated annually on July 1 on the basis of the gross national income per capita data from the previous calendar year. Second, information about when the Delta variant became dominant in each country was gleaned from SARS-CoV-2 genomic sequences published in the GISAID (18) and GenBank (19) databases. Last, we used Our World in Data (OWID) (15,20) to obtain data on confirmed cases, deaths, percentage of the population fully vaccinated, percentage of the population >65 years of age, population density, SARS-CoV-2 testing volume, and stringency (a composite index of 9 mitigation policies and strategies). OWID compiles data from Johns Hopkins University, World Bank, national government reports, and Oxford University. Data were reported daily (confirmed cases and deaths, stringency index), every weekday (vaccinations), weekly (testing), or annually (population >65 years of age, population density) during the period of interest.
Data Management
We downloaded sequence data from GISAID and GenBank, aggregated at the country-level, and removed duplicate sequences that were in both databases. Then, for each country, we calculated the weekly proportions of Delta variant sequences during the period of interest, April 1, 2020–November 30, 2021.
To enable easy comparisons between the periods before and after the emergence of the Delta variant, we defined the Delta dominance date as the Monday of the first week in which >50% of sequenced samples were Delta. We defined the pre-Delta period as April 1, 2020, to the Delta dominance date and the Delta dominance period as the Delta dominance date through November 30, 2021.
Outcome and Predictor Variables
We used measures of illness and death as outcomes. For the pre-Delta and Delta dominance periods, we calculated the peak 7-day rolling averages for case rates, death rates, and case-fatality rates (CFRs) per million persons. We chose the 7-day peak rolling average to indicate the intensity of the outbreak while also minimizing the effect of bulk data uploads. CFRs used a 14-day lag between daily new cases and daily new deaths (21) and was calculated as a ratio of reported deaths to reported cases. Death rates and CFRs offer different types of information about deaths: death rates account for the probability of death in an entire population, whereas CFRs only measure the probability of death among those with the disease. Adherence to case and death reporting might vary by country. To standardize outcomes across countries, we calculated a Delta dominance to pre-Delta ratio (DD:PD) for each outcome. We calculated 3 outcomes of interest as a 7-day rolling average: peak case rate DD:PD, peak death rate DD:PD, and peak CFR DD:PD.
We classified outcome variables into quartiles to enable easier interpretation and statistical evaluation of small datasets. For example, the outcome variable peak case rate DD:PD predictor variables included the percent of the total population fully vaccinated with a primary series at the date of Delta dominance, the percentage of the population >65 years of age, and population density (persons/km2). We also included the median 7-day rolling average daily testing volume DD:PD (comparing the period of Delta predominance to the pre-Delta period, as described). Last, we calculated the median stringency index DD:PD. The stringency index, as defined by other scholars (22), ranges from 0–100 (100 = strictest) and increases over time if more stringent mitigation policies are implemented or decreases if policies are rescinded (23).
Statistical Analysis
We managed and analyzed data using SPSS Statistics 1.0.0.1406 (IBM Corp., https://www.ibm.com) and RStudio 1.4.1717 (PBC, https://posit.co/blog/rstudio-pbc). To characterize the speed at which Delta supplanted previous variants, we calculated the number of days from the date of the first Delta sequence collection to the date of Delta dominance and from the start of Delta dominance to peak case and death rates (Figure 1). We calculated descriptive statistics (range, mean, median, peak, quartiles) for all measures of illness and death and predictor variables during the pre-Delta and Delta dominance periods.
We used Spearman rank-order correlation to assess the strength and direction of the association between quartile outcomes with predictor variables. For bivariate correlations with p values <0.25, we further assessed relationships between outcomes and predictors in multivariable ordinal regression models. Each of the outcomes were modeled separately. We explored all possible combinations of predictor variables meeting the above criteria and selected the model with the lowest Akaike Information Criterion score as the best model. We evaluated multicollinearity among the predictor variables using a Condition Index cutoff of <15. This activity was reviewed by Centers for Disease Control and Prevention and conducted consistent with applicable federal law and CDC policy (see, e.g., 45 C.F.R. part 46.102(l) (2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Descriptive Analysis
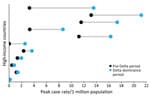
Among 79 HICs, 45 met the inclusion criteria. The first Delta sequence collection dates ranged from December 28, 2020, to July 26, 2021; more than half (n = 25, 55%) of the countries reported a Delta sequence by the end of March 2021. The median time between the first Delta sequence collected to the start of the Delta dominance period was 77 days (interquartile range [IQR] 49–140). The median time from the start of the Delta dominance period to the peak case rates was 144 (IQR 65–155) days and to the peak death rate was 141 (IQR 70–155) days (Figure 2). Weekly case incidence during the Delta dominance period varied by country and WHO region (Appendix Figure). Average peak case rates ranged from 180 in the WHO Western Pacific region to 1,699 in the African region.
Most countries (57%, n = 26) reported higher peak case rates (Figure 3) but lower peak CFRs (98%, n = 44) and peak death rates (75%, n = 34) (Figure 4) during the Delta dominance period than during the pre-Delta period. Ten (22%) countries reported both higher peak case rates and death rates during the Delta dominance period than during the pre-Delta period.
Vaccination coverage with a primary series at the start date of Delta dominance ranged from 1% to 72% in 44 of 45 countries for which data were available (Table 1). The percentage of the population >65 years of age averaged 15.7% (IQR 13.9%–19.5%) in 44 countries for which data were available. Most countries (84%, n = 38) had a lower stringency index during the Delta dominance period than during the pre-Delta period.
Peak Case Rate DD:PD Ratio
Higher vaccination coverage was protective against higher case rates; that is, vaccination resulted in lower peak case rate DD:PD ratios (Spearman rank correlation coefficient [ρ] = −0.36; p = 0.018) (Table 2). The same association was held in the ordinal regression model (odds ratio [OR] 0.95, 95% CI 0.91–0.99; p = 0.01) (Tables 3, 4). There was a positive correlation between median daily testing volume and peak case rate DD:PD ratios (Spearman ρ = 0.30; p = 0.048) (Table 2), although that relationship did not hold in the ordinal regression models (Table 4).
Peak Death Rate DD:PD Ratio
A higher percentage of the population being persons >65 years of age was protective against death rates (peak death rate DD:PD ratios), both in bivariate comparisons (Spearman ρ = −0.31; p = 0.039) (Table 2) and in the regression models (OR 0.88, 95% CI 0.78–0.98; p = 0.02). In addition, higher vaccination coverage was associated with lower peak death rate DD:PD ratios (OR 0.96, 95% CI 0.92–0.99; p = 0.03) in the regression models.
Peak CFR DD:PD Ratio
A higher percentage of the population being persons >65 years of age was associated with lower CFRs (lower peak CFR DD:PD ratios, Spearman ρ = −0.48; p = 0.001) (Table 2). This association held in the ordinal regression model (OR 0.89, 95% CI 0.80–0.98; p = 0.01).
Most HICs had higher case rates but lower death rates and CFRs during the Delta dominance period than in their pre-Delta periods. Achieving higher vaccination coverage appeared to be the main public health measure associated with a decrease in the intensity (peak) of COVID-19 cases and deaths. Each quartile increase in vaccination coverage resulted in a 5% reduction in peak case rates and a 4% reduction in peak death rates. Countries with a larger percentage of persons >65 years of age had lower death rates and CFRs during the Delta dominance period, likely because of focused early vaccination activities in this age group and case management protocols prioritizing older populations for close observation and hospital admission (24,25).
Many HICs recorded increased case rates during the Delta dominance period, but those increases did not often result in higher death rates than for the pre-Delta period. Previous studies reported mixed results on death rates during the period of Delta variant prevalence; 2 studies reported no differences in mortality rates (26) or in-hospital deaths (27), and 3 studies indicated an increase in mortality rates (28; A. Kumar et. Al, unpub. Data, https://www.medrxiv.org/content/10.1101/2021.09.23.21263948v1) and in-hospital deaths (R.S. Khedar et al., unpub. Data, https://www.medrxiv.org/content/10.1101/2021.09.03.21263091v1). The lower death rates and CFRs among HICs in this study may be the result of advances in therapeutics, improvements in case management protocols, increased natural immunity from previous infection, and the availability of vaccines, rather than decreased virulence of the Delta variant virus (29–31). Among global studies from HICs, the early prioritization of vaccination in the elderly and achievement of high coverage appeared to protect older adults against death during the Delta dominance period. Studies have shown that, during the Delta wave, vaccines were less effective against infection (32,33; R.S. Barlow et al., unpub. data, https://www.medrxiv.org/content/10.1101/2021.08.30.21262446v1) but highly protective against symptomatic disease (29,33), hospitalization (34), and death (33). This lack of protection against infection among vaccinated persons, combined with the increased transmissibility of the Delta variant, likely contributed to an overall higher case burden during the Delta predominance period in HICs. Nevertheless, despite Delta’s high transmissibility, peak case and death rates occurred in many countries >4 months after the date of Delta’s dominance, which would have allowed time for strategies such as vaccination to be implemented and have an effect (35).
The first limitation of this study is that we used publicly available datasets, which might be affected by fluctuations in reporting, including reporting of at-home testing. Death rates might not be a robust indicator of severity in HICs. Hospitalization data might have provided another measure of illness severity, but OWID hospitalization data were available for <50% of countries included in the study and had many reporting gaps. The goal of this study was to identify population-level associations between COVID-19 illness and death and disease control metrics; a priori testing of more specific hypotheses might be addressed in other assessments using different study designs (e.g., a community-wide, cluster-randomized trial that evaluated masking in Bangladesh [36]). This analysis was ecologic in nature, and therefore population-level associations might not apply at the individual level. We also focused on the outcomes of peak cases, deaths, and CFRs and therefore did not capture the total burden of cases and deaths. Finally, the variety of COVID-19 vaccines available and changing recommendations on the number of doses for maximal effectiveness make it difficult to generalize the findings associated with vaccination coverage to all COVID-19 variants and coverage effectiveness over time.
In conclusion, this characterization of epidemiologic outcomes in high-income countries during the Delta dominance period shows that many countries reported higher case rates but lower death and case fatality rates compared to the pre-Delta period; higher vaccination coverage and completion of a primary vaccination series were associated with lower case and death rates; and >4 months elapsed between Delta introduction and peaks in case rates and death rates, which might have allowed time for mitigation strategies such as vaccination to be implemented and have an impact. These findings might be useful in informing public health authorities of the importance of achieving high vaccination coverage as the pandemic continues to evolve. The ability to continue implementing measures to combat COVID-19 might be limited by authorities’ willingness to implement stronger measures and the willingness of the public to comply. However, across multiple HICs, our findings consistently indicate that higher vaccination coverage can result in fewer cases and deaths.
Dr. Atherstone is an epidemiologist with broad experience in zoonotic infectious disease surveillance, outbreak response, and research in the United States and sub-Saharan Africa. She has expertise working in multiple countries, alongside dozens of partners, in remote, difficult conditions implementing complex investigations.
Acknowledgment
We thank Barbara Marston, Zachary Myles, David Shih, and Xinjian Zhang for their contributions to this research.
References
- World Health Organization. Tracking SARS-CoV-2 variants [cited 2022 Jan 26]. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants
- GISAID. Tracking of hCoV-19 variants. 2020 [cited 2022 Jan 26]. https://www.gisaid.org/hcov19-variants
- World Health Organization. COVID-19 vaccination data. 2022 [cited 2022 Jan 26]. https://covid19.who.int/who-data/vaccination-data.csv "https://covid19.who.int/"
- Our World in Data. COVID-19 data explorer [cited 2022 Jan 26]. https://ourworldindata.org/explorers/coronavirus-data-explorer
- New Zealand Ministry of Health. COVID-19 community response framework. 2022 [cited 2022 Apr 14]. https://www.health.govt.nz/covid-19-novel-coronavirus/covid-19-response-planning/covid-19-community-response-framework
- Soo Z, Fung A. Hong Kong orders mandatory COVID-19 tests for all residents. Associated Press. 2022 Feb 22 [cited 2022 Apr 14]. https://apnews.com/article/coronavirus-pandemic-health-hong-kong-4bb8dd1225887f06f5a0d981a4b52505
- Chen J-M, Chen Y-Q. China can prepare to end its zero-COVID policy. Nat Med. 2022;28:1104–5. DOIPubMedGoogle Scholar
- World Health Organization. Statement on Omicron sublineage BA.2. 2022 Feb 22 [cited 2022 Apr 13]. https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2
- Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323:1915–23. DOIPubMedGoogle Scholar
- Dehning J, Zierenberg J, Spitzner FP, Wibral M, Neto JP, Wilczek M, et al. Inferring change points in the spread of COVID-19 reveals the effectiveness of interventions. Science. 2020;369:
eabb9789 . DOIPubMedGoogle Scholar - Medline A, Hayes L, Valdez K, Hayashi A, Vahedi F, Capell W, et al. Evaluating the impact of stay-at-home orders on the time to reach the peak burden of Covid-19 cases and deaths: does timing matter? BMC Public Health. 2020;20:1750. DOIPubMedGoogle Scholar
- Fuller JA, Hakim A, Victory KR, Date K, Lynch M, Dahl B, et al.; CDC COVID-19 Response Team. Mitigation policies and COVID-19–associated mortality—37 European countries, January 23–June 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:58–62. DOIPubMedGoogle Scholar
- Smith DJ, Hakim AJ, Leung GM, Xu W, Schluter WW, Novak RT, et al. COVID-19 mortality and vaccine coverage—Hong Kong Special Administrative Region, China, January 6, 2022–March 21, 2022. China CDC Wkly. 2022;4:288–92. DOIPubMedGoogle Scholar
- Talic S, Shah S, Wild H, Gasevic D, Maharaj A, Ademi Z, et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. BMJ. 2021;375:
e068302 . DOIPubMedGoogle Scholar - Hasell J, Mathieu E, Beltekian D, Macdonald B, Giattino C, Ortiz-Ospina E, et al. A cross-country database of COVID-19 testing. Sci Data. 2020;7:345. DOIPubMedGoogle Scholar
- Ohlsen EC, Hawksworth AW, Wong K, Guagliardo SAJ, Fuller JA, Sloan ML, et al. Determining gaps in publicly shared SARS-CoV-2 genomic surveillance data by analysis of global submissions. Emerg Infect Dis. 2022;28:S85–92. DOIPubMedGoogle Scholar
- The World Bank. World Bank country and lending groups [cited 2021 Nov 30]. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Elbe S, Buckland-Merrett G. Data, disease and diplomacy: GISAID’s innovative contribution to global health. Glob Chall. 2017;1:33–46. DOIPubMedGoogle Scholar
- Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. GenBank. Nucleic Acids Res. 2016;44(D1):D67–72. DOIPubMedGoogle Scholar
- Mathieu E, Ritchie H, Ortiz-Ospina E, Roser M, Hasell J, Appel C, et al. A global database of COVID-19 vaccinations. Nat Hum Behav. 2021;5:947–53. DOIPubMedGoogle Scholar
- Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. DOIPubMedGoogle Scholar
- Hale T, Angrist N, Goldszmidt R, Kira B, Petherick A, Phillips T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5:529–38. DOIPubMedGoogle Scholar
- Blavatnik School of Government. Coronavirus government response tracker [cited 2021 Dec 7]. https://www.bsg.ox.ac.uk/research/research-projects/coronavirus-government-response-tracker
- Ministry of Health and Family Welfare, Government of India. Clinical guidance for management of adult COVID-19 patients [cited 2022 May 30]. https://www.mohfw.gov.in/pdf/ClinicalGuidanceforManagementofAdultCOVID19Patientsupdatedason05thjan2023.pdf
- World Health Organization. COVID-19 clinical management: living guidance. 2021 Jan 25 [cited 2022 May 30]. https://apps.who.int/iris/handle/10665/338882
- Christensen PA, Olsen RJ, Long SW, Subedi S, Davis JJ, Hodjat P, et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am J Pathol. 2022;192:320–31. DOIPubMedGoogle Scholar
- Taylor CA, Patel K, Pham H, Whitaker M, Anglin O, Kambhampati AK, et al.; COVID-NET Surveillance Team. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B. 1.617. 2 (Delta) predominance—COVID-NET, 14 states, January–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1513–9. DOIPubMedGoogle Scholar
- Fisman DN, Tuite AR. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ. 2021;193:E1619–25. DOIPubMedGoogle Scholar
- Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;385:585–94. DOIPubMedGoogle Scholar
- Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, et al.; MSHI. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of Delta and Omicron variant emergence—25 US jurisdictions, April 4–December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132–8. DOIPubMedGoogle Scholar
- Saban M, Myers V, Wilf-Miron R. Changes in infectivity, severity and vaccine effectiveness against delta COVID-19 variant ten months into the vaccination program: The Israeli case. Prev Med. 2022;154:
106890 . DOIPubMedGoogle Scholar - Sheikh A, McMenamin J, Taylor B, Robertson C; Public Health Scotland and the EAVE II Collaborators. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2. DOIPubMedGoogle Scholar
- Ng OT, Koh V, Chiew CJ, Marimuthu K, Thevasagayam NM, Mak TM, et al. Impact of Delta variant and vaccination on SARS-CoV-2 secondary attack rate among household close contacts. Lancet Reg Health West Pac. 2021;17:
100299 . DOIPubMedGoogle Scholar - Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al.; VISION Network. VISION Network. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B. 1.617. 2 (Delta) variant predominance—nine states, June–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1291–3. DOIPubMedGoogle Scholar
- Li H, Wang L, Zhang M, Lu Y, Wang W. Effects of vaccination and non-pharmaceutical interventions and their lag times on the COVID-19 pandemic: Comparison of eight countries. PLoS Negl Trop Dis. 2022;16:
e0010101 . DOIPubMedGoogle Scholar - Abaluck J, Kwong LH, Styczynski A, Haque A, Kabir MA, Bates-Jefferys E, et al. Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Science. 2022;375:
eabi9069 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: July 26, 2023
Table of Contents – Volume 29, Number 9—September 2023
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Christine Atherstone, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-12, Atlanta, GA 30329-4027, USA
Top