Sensitivity to Neutralizing Antibodies and Resistance to Type I Interferons in SARS-CoV-2 R.1 Lineage Variants, Canada
Rajesh Abraham Jacob, Ali Zhang
1, Hannah O. Ajoge
1, Michael R. D'Agostino
1, Kuganya Nirmalarajah, Altynay Shigayeva, Wael L. Demian, Sheridan J.C. Baker, Hooman Derakhshani, Laura Rossi, Jalees A. Nasir, Emily M. Panousis, Ahmed N. Draia, Christie Vermeiren, Jodi Gilchrist, Nicole Smieja, David Bulir, Marek Smieja, Michael G. Surette, Andrew G. McArthur, Allison J. McGeer, Samira Mubareka, Arinjay Banerjee, Matthew S. Miller, and Karen Mossman
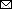
Author affiliations: McMaster University, Hamilton, Ontario, Canada (R.A. Jacob, A. Zhang, H.O. Ajoge, M.R. D'Agostino, W.L. Demian, S.J.C. Baker, L. Rossi, J.A. Nasir, E.M. Panousis, A.N. Draia, D. Bulir, M. Smieja, M.G. Surette, A.G. McArthur, M.S. Miller, K. Mossman); Sunnybrook Research Institute, Toronto, Ontario, Canada (K. Nirmalarajah, S. Mubareka); University of Toronto, Toronto (A. Shigayeva, C. Vermeiren, A.J. McGeer, S. Mubareka, A. Banerjee); University of Manitoba, Winnipeg, Manitoba, Canada (H. Derakhshani); Research Institute of St. Joe’s Hamilton, Hamilton (J. Gilchrist, N. Smieja, D. Bulir); Vaccine and Infectious Disease Organization, Saskatoon, Saskatchewan, Canada (A. Banerjee); University of Saskatchewan, Saskatoon (A. Banerjee); University of Waterloo, Waterloo, Ontario, Canada (A. Banerjee); University of British Columbia, Vancouver, British Columbia, Canada (A. Banerjee)
Main Article
Figure 4

Figure 4. Resistance to type I interferons in SARS-CoV-2 R.1 lineage variants, Canada. A) Sensitivity of SB3, R.1 645, and R.1 646 to IFN-α. B) Sensitivity of SB3, R.1 645, and R.1 646 to IFN-β. C, D) Fold change in IFIT1 transcript levels in response to IFN-α (C) or IFN-β (D) treatment. E, F) Fold change in IRF7 transcript levels in response to IFN-α (E) or IFN-β treatment (F). ISG transcript levels were normalized to GAPDH transcript levels. For testing, Calu-3 cells were either mock-infected or infected with SARS-CoV-2 (50,000 PFU/well) for 1 hour followed by treatment with recombinant IFN (1 or 10 ng/mL). Total RNA was extracted after 72 hours and SARS-CoV-2 RNA levels were determined using quantitative reverse transcription PCR. 1/ΔCT values are represented after normalizing to mock-infected cells. Statistical significance was calculated using 2-way analysis of variance with Tukey multiple comparisons test. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFIT1, interferon-induced protein with tetratricopeptide repeats; IFN, interferon; IRF7, interferon regulatory factor 7; ISG, IFN-stimulated gene.
Main Article
Page created: May 08, 2023
Page updated: June 20, 2023
Page reviewed: June 20, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
