At a glance
- An updated list of high-risk underlying conditions, along with their associated evidence, is provided below. The conditions are grouped by the level of evidence, with the highest level shown in the top section.
- The list of underlying medical conditions is not exhaustive and will be updated as the science evolves.
- This list should not be used to exclude people with underlying conditions from recommended measures for prevention or treatment of COVID-19.
What you need to know
This web page provides an evidence-based resource for healthcare professionals caring for patients with underlying medical conditions who are at higher risk of experiencing severe outcomes of COVID-19. Severe outcomes of COVID-19 are defined as hospitalization, admission to the intensive care unit (ICU), intubation or mechanical ventilation, or death.
Information for the General Public
This page summarizes data from published reports, scientific articles in press, unreviewed pre-prints, and internal data that were included in literature reviews conducted by subject matter experts. Evidence used to inform the list of underlying conditions was determined by CDC reviewers based on available literature about COVID-19 at time of review. The information reflects evidence regarding underlying medical conditions and is intended to help healthcare professionals make informed decisions about patient care and to increase the awareness of risk among their patients.
The methods used to assess the conditions have changed during the pandemic as the amount of literature and types of studies increased. For instance, preliminary versions of this list focused on providing the latest information based on descriptive data. As the literature grew, CDC investigators categorized the literature by study design.
Since May 2021, the process has been updated to include a CDC-led review process that uses rigorous systematic review methods. To learn more about the process of CDC’s systematic reviews, see CDC systematic review process.
Background
Age is the strongest risk factor for severe COVID-19 outcomes. Patients with one or multiple certain underlying medical conditions are also at higher risk.(1-3)
Additionally, being unvaccinated or not being up to date on COVID-19 vaccinations also increases the risk of severe COVID-19 outcomes.
Providers should consider the patient's age, presence of underlying medical conditions and other risk factors, and vaccination status in determining the risk of severe COVID-19-associated outcomes for any patient.
Demographic Factors
Studies have shown that COVID-19 does not affect all population groups equally. Three important factors are age, race, and ethnicity.
Age
Age remains the strongest risk factor for severe COVID-19 outcomes, with risk of severe outcomes increasing markedly with increasing age. Based on data from the National Vital Statistics System (NVSS) at NCHS (Risk for COVID-19 Infection, Hospitalization, and Death By Age Group), compared with ages 18–29 years, the risk of death is 25 times higher in those ages 50–64 years, 60 times higher in those ages 65–74 years, 140 times higher in those ages 75–84 years, and 340 times higher in those ages 85+ years. Notably, these data include all deaths in the United States that occurred throughout the pandemic, from February 2020 to July 1, 2022, including deaths among unvaccinated individuals.
Risk of severe outcomes is increased in people of all ages with certain underlying medical conditions and in people who are 50 years and older, with risk increasing substantially at ages >65 years.4,5 Residents of long-term care facilities are also at increased risk, making up less than 1% of the U.S. population but accounting for more than 35% of all COVID-19 deaths.6-10
Race and Ethnicity
The COVID-19 pandemic has highlighted racial, ethnic, and socioeconomic disparities in COVID-19 illnesses, hospitalizations, and deaths.11-13 Some racial and ethnic minority groups are also more likely to face multiple barriers to accessing health care including lack of insurance, transportation, child care, or ability to take time off from work.
Studies have identified racial and ethnic differences in at-home COVID-19 test use, vaccination coverage, and access to outpatient therapeutics.14-16 Data has shown that compared to non-Hispanic White people, people from racial and ethnic minority groups are more likely to be infected with SARS-CoV-2 (the virus that causes COVID-19). Once infected, people from racial and ethnic minority groups are more likely to be hospitalized, be admitted to the ICU, and die from COVID-19 at younger ages.17
We are still learning about how the environments where people live, learn, and work can influence the risk for infection and severe COVID-19 outcomes.
Summary of Conditions with Evidence
Evidence used to inform the list of underlying medical conditions that increase a person's risk of severe illness from COVID-19 is presented in alphabetical order by study design section. Conditions are categorized as higher risk, suggestive higher risk, and mixed evidence.
Higher Risk (conclusive)
Higher risk is defined as an underlying medical condition or risk factor that has a published meta-analysis or systematic review or underwent the CDC systematic review process. The meta-analysis or systematic review demonstrates a conclusive increase in risk for at least one severe COVID-19 outcome.
Notice
* Indicates presence of evidence for pregnant and non-pregnant people
‡ Underlying conditions for which there is evidence in pediatric patients
^ Risk may be further increased for people receiving dialysis
Condition
Evidence of Impact on COVID-19 Severity [Reference number]
Asthma
CDC Systematic Review [K]
Cancer
- Hematologic Malignancies
CDC Systematic Review [O]
Meta-Analysis/ Systematic Review 18-22
Cohort Study 23-25
Case Series 26-28
Case Control Study 29
Cerebrovascular disease
Meta-Analysis 30-33
Synthesis of Evidence 34
Cohort Study 35-37
Chronic kidney disease*
- People receiving dialysis 38,39 ^
Meta-Analysis 33,40
Cohort Studies 36,41-62, 63*
Case Series 64-66
Chronic lung diseases limited to:
- Bronchiectasis
- COPD (Chronic obstructive pulmonary disease)
- Interstitial lung disease
- Pulmonary embolism
- Pulmonary hypertension
- CDC Systematic Review [A]
- CDC Systematic Review [L]
- CDC Systematic Review [D]
- CDC Systematic Review [G]
- CDC Systematic Review [G]
Chronic liver diseases limited to:
- Cirrhosis
- Non-alcoholic fatty liver disease
- Alcoholic liver disease
- Autoimmune hepatitis
CDC Systematic Review [B] 211
Cystic fibrosis
CDC Systematic Review [M]
Diabetes mellitus, type 1
Meta-Analysis 67
Case Series 65
Cohort Study 35,68-73
Diabetes mellitus, type 2*
Meta-Analysis 74
Systematic Review 75*
Gestational Diabetes Systematic Review 76*
Case Series 65
Longitudinal Study 77
Cohort Study 67,71,77-82
Disabilities‡, including Down syndrome
For the list of all conditions that were part of the review, see the module below
CDC Systematic Review [C]
Heart conditions (such as heart failure, coronary artery disease, or cardiomyopathies)
Meta-Analysis 83-85
Cohort Study 35,36
HIV (Human immunodeficiency virus)
Meta-Analysis/ Systematic Review 86
Cohort Study 54,87-89
Case Series 90-92
Mental health conditions limited to:
- Mood disorders, including depression
- Schizophrenia spectrum disorders
Meta-Analysis/ Systematic Review 93,94
Neurologic conditions limited to dementia‡ and Parkinson’s Disease
Meta-Analysis/ Systematic Review 95-98, 208
Cohort Study 36
Obesity (BMI >30 kg/m2 or >95th percentile in children)
Meta-Analysis 101-103
Systematic Review 75*
Cohort 46,104-112,63,113-116*
Physical inactivity
CDC Systematic Review [E]
Pregnancy and recent pregnancy
Meta-Analysis/ Systematic Review 75,117
Case Control 118,119
Case Series 120-122
Cohort Study 123-126
Primary immunodeficiencies
CDC Systematic Review [F]
Smoking, current and former
Meta-Analysis 83,127,128-135
Solid organ or blood stem cell transplantation
Meta-Analysis 108
Case Series 136-147
Cohort 148-151
Tuberculosis
CDC Systematic Review [H]
Use of corticosteroids or other immunosuppressive medications
Meta-Analysis/ Systematic Review 152
Cohort Study 153
Cross-Sectional 154
Case Series 155-157
Complete List of Disabilities from CDC’s Systematic Review Process
- Attention-deficit/hyperactivity disorder (ADHD)
- Autism
- Cerebral palsy
- Charcot foot
- Chromosomal disorders
- Chromosome 17 and 19 deletion
- Chromosome 18q deletion
- Cognitive impairment
- Congenital hydrocephalus
- Congenital malformations
- Deafness/hearing loss
- Disability indicated by Barthel Index
- Down syndrome
- Fahr's syndrome
- Fragile X syndrome
- Gaucher disease
- Hand and foot disorders
- Learning disabilities
- Leber's hereditary optic neuropathy (LHON) or Autosomal dominant optic atrophy (ADOA)
- Leigh syndrome
- Limitations with self-care or activities of daily living
- Maternal inherited diabetes and deafness (MIDD)
- Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) and risk markers
- Mobility disability
- Movement disorders
- Multiple disability (referred to in research papers as "bedridden disability")
- Multisystem disease
- Myoclonic epilepsy with ragged red fibers (MERRF)
- Myotonic dystrophy
- Neurodevelopmental disorders
- Neuromuscular disorders
- Neuromyelitis optica spectrum disorder (NMOSD)
- Neuropathy, ataxia, and retinitis pigmentosa (NARP)
- Perinatal spastic hemiparesis
- Primary mitochondrial myopathy (PMM)
- Progressive supranuclear palsy
- Senior-Loken syndrome
- Severe and complex disability (referred to in research papers as "polyhandicap disability")
- Spina bifida and other nervous system anomalies
- Spinal cord injury
- Tourette syndrome
- Traumatic brain injury
- Visual impairment/blindness
- Wheelchair use
Suggestive Higher Risk
Suggestive higher risk is defined as an underlying medical condition or risk factor that did not have a published meta-analysis or systematic review or did not undergo the CDC systematic review process. The evidence is supported by mostly cohort, case-control, or cross-sectional studies. (Systematic reviews are available for some conditions for children with underlying conditions.)
Condition
Evidence of Impact on COVID-19 Severity [Reference number]
Children with certain underlying conditions
Systematic Review 158,159
Cross-Sectional Study 99,160,161
Cohort Study 99,100,162-169
Case Series 170,171
Epilepsy
Cohort Study209
Hemophilia
Cohort Study210
Overweight (BMI >25 kg/m2 but <30 kg/m2)
Cohort Study111
Case Series110
Sickle cell disease
Cohort170-173
Case Series 170,173-188
Substance use disorders
Case-Control Study 189-191
Cohort Study 192,193
Mixed Evidence (inconclusive: no conclusions can be drawn from the evidence)
Mixed evidence is defined as an underlying medical condition or risk factor that has a published meta-analysis or systematic review or underwent the CDC systematic review process. The meta-analysis or systematic review is inconclusive, either because the aggregated data on the association between an underlying condition and severe COVID-19 outcomes are inconsistent in direction or there are insufficient (or limited) data on the association between an underlying condition and severe COVID-19 outcomes.
- Limited: The evidence consists of one study, or several small studies with no comparison group, limiting the conclusions that can be drawn.
- Inconsistent: The evidence suggests no clear direction of association, meaning no firm conclusions can be drawn.
Notice
* Indicates presence of evidence for pregnant and non-pregnant people
‡ Underlying conditions for which there is evidence in pediatric patients
^ Risk may be further increased for people receiving dialysis
Condition
Evidence of Impact on COVID-19 Severity [Reference number]
Alpha 1 antitrypsin deficiency
Limited: CDC Systematic Review [I]
Bronchopulmonary dysplasia
Limited: CDC Systematic Review [J]
Hepatitis B
Inconsistent: CDC Systematic Review [B]
Hepatitis C
Limited: CDC Systematic Review [B]
Hypertension*
Inconsistent
Meta-Analysis 83,194-197
Systematic Review 198, 75*
Cohort Study 35,36,41,199-205
Case Series 206
Thalassemia
Limited: CDC Systematic Review [N]
Actions Healthcare Professionals Can Take
- Recommend vaccination with approved and authorized COVID-19 vaccines (updated 2024-2025 COVID-19 vaccine), which are safe and effective. Check out the Interim Clinical Considerations for Use of COVID-19 Vaccines as well as Stay Up to Date with Your Vaccines and locations for COVID-19 vaccination for patients for more information.
- Prescribe antivirals, which have been shown to significantly decrease the risk of hospitalization and death when treating patients with mild or moderate illness and risk factors for severe illness. Outcomes are improved if therapeutics are started within the first 5-7 days of symptom onset.
- Consider Pemivibart (Pemgarda™), a monoclonal antibody for COVID-19 pre-exposure prophylaxis in people who are moderately or severely immunocompromised and unlikely to mount an adequate immune response to COVID-19 vaccination and who meet the FDA-authorized conditions for use. Pemivibart is an IV-infusion monoclonal antibody that is authorized for pre-exposure prevention of COVID-19 for individuals (12 years of age and older weighing at least 40 kg). Pemivibart may provide another layer of protection against COVID-19 in addition to vaccination and can be given at least 2 weeks after receiving a COVID-19 vaccine. Healthcare providers should consult the Pemivibart EUA Fact Sheet and EUA Frequently Asked Questions for the FDA-authorized conditions under which Pemivibart may be used. CDC is monitoring variants and how commonly they occur to understand if they might affect how well Pemivibart works. The FDA will provide additional updates to the EUA materials, as appropriate, if new information emerges. This is the only preventive option available for COVID-19 for the immunocompromised community, as described above, at the present time.
- Remind older patients and those with underlying medical conditions that wearing a mask is an additional prevention strategy they can choose to further protect themselves.
- Encourage patients to keep appointments for routine care and adhere to treatment regimens for their medical conditions.
- Consider use of telehealth when appropriate.
- Check out additional information for your patients.
Considerations for Patients Within Racial and Ethnic Minority Groups
- Ask patients about their concerns about vaccines and therapy. Consider using an evidence-based and culturally sensitive approach, such as motivational interviewing. Try to provide trusted sources of information and other resources.
- Encourage nucleic acid amplification tests (NAATs), including PCR tests, as well as early treatment for patients who are eligible.
- Facilitate access to culturally and linguistically appropriate resources.
- Reduce barriers to accessing current outpatient treatments.
CDC strongly encourages healthcare professionals, patients and their advocates, and health system administrators to regularly consult the Infectious Diseases Society of America (IDSA) COVID-19 Treatment Guidelines.
Key Findings from One Large Cross-Sectional Study
Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021
This study used data from the Premier Healthcare Database, which represents approximately 20% of all inpatient admissions in the United States since 2000. This cross-sectional study of 540,667 adults hospitalized with COVID-19 included both inpatients and hospital-based outpatients with laboratory-diagnosed COVID-19 from March 1, 2020, through March 31, 2021. The database included reports from 592 acute care hospitals in the United States. The study was designed to examine risk factors associated with severe outcomes of COVID-19 including admission to an ICU or stepdown unit, invasive mechanical ventilation, and death.
Main Findings:
- Certain underlying medical conditions were associated with an increased risk for severe COVID-19 illness in adults.
- Having multiple conditions was also associated with severe COVID-19 illness.
- Obesity, diabetes with complications, and anxiety and fear-related disorders had the strongest association with death.
- The number of frequent underlying medical conditions (present in ≥10.0% of patients) increased with age.207
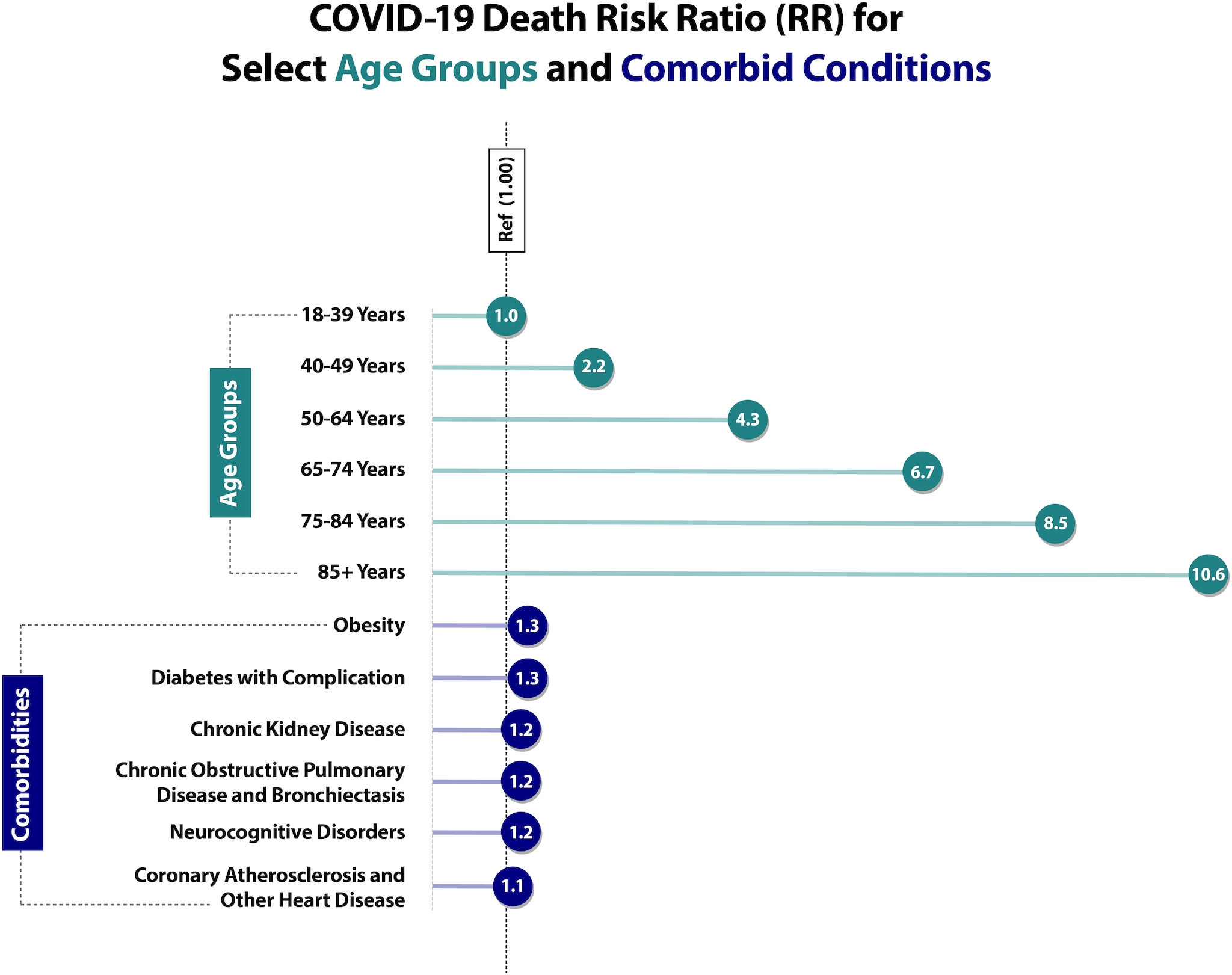
Adapted from Sources:
- Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. To learn more, visit the Preventing Chronic Disease article: https://www.cdc.gov/pcd/issues/2021/21_0123.htm
- Pennington AF, Kompaniyets L, Summers AD, Danielson ML, Goodman AB, Chevinsky JR, Preston LE, Schieber LZ, Namulanda G, Courtney J, Strosnider HM, Boehmer TB, Mac Kenzie WR, Baggs J, Gundlapalli AV, Risk of Clinical Severity by Age and Race/Ethnicity Among Adults Hospitalized for COVID-19—United States, March–September 2020, Open Forum Infectious Diseases, Volume 8, Issue 2, February 2021. To learn more, visit: https://doi.org/10.1093/ofid/ofaa638
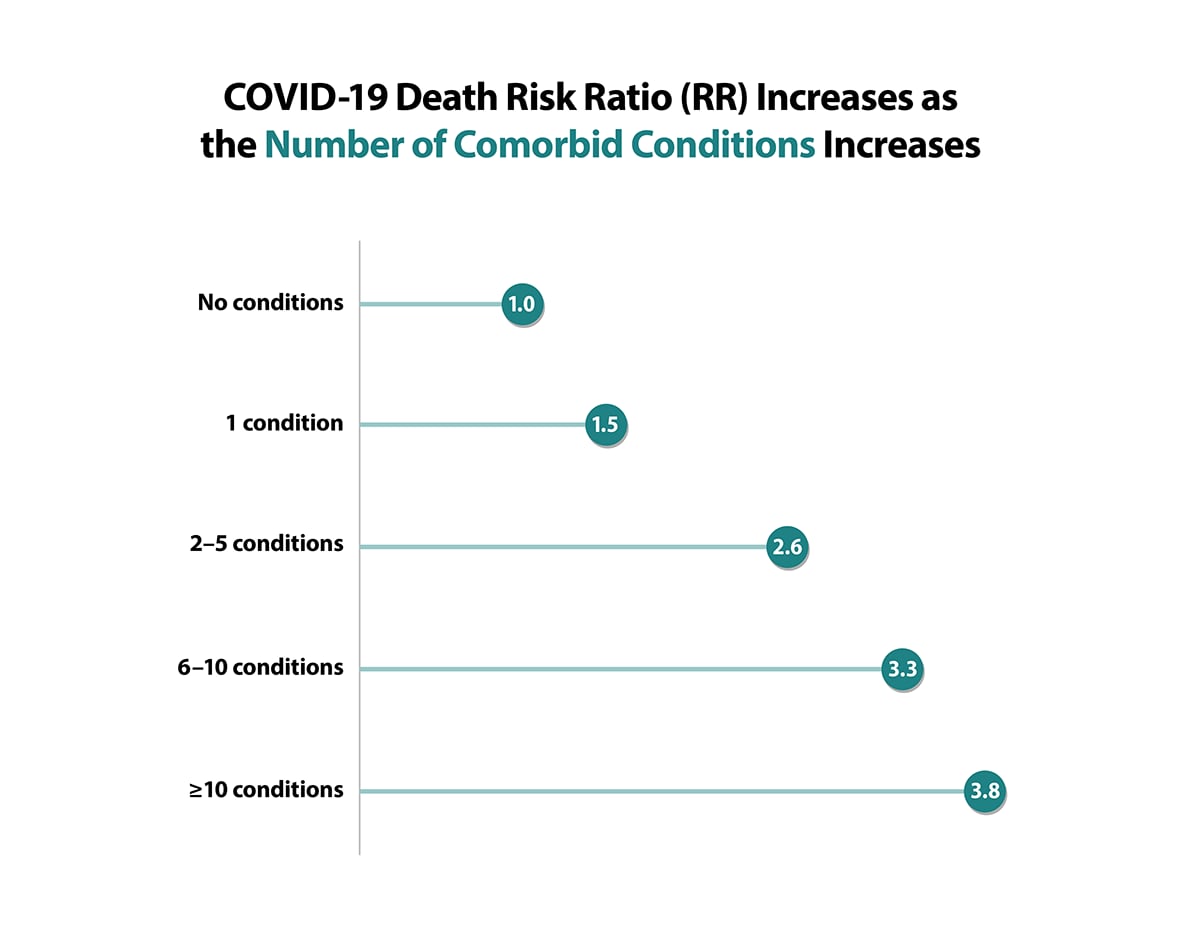
Source: Kompaniyets L, Pennington AF, Goodman AB, Rosenblum HG, Belay B, Ko JY, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020–March 2021. To learn more, visit the Preventing Chronic Disease article: https://www.cdc.gov/pcd/issues/2021/21_0123.htm
More Information
- Methods for the Underlying Conditions ICD-10 List [PDF, 2 pages, 112K]
- COVID-19 Therapeutics
- Clinical Presentation
- COVID-19 Treatment in Outpatients
- United States COVID-19 Deaths, Emergency Department (ED) Visits, and Test Positivity by Geographic Area
- Demographic Trends of COVID-19 Deaths in the US Reported to NVSS
- Health Equity: Promoting Fair Access to Health
- COVID-19 Vaccination Clinical & Professional Resources
References
See CDC Systematic Review References
- Stone EC, Weissman D, Mazurek J, et al. Brief Summary of Findings on the Association Between Underlying Bronchiectasis and Severe COVID-19 Outcomes. [print only, 476K, 18 pages]CDC COVID-19 Scientific Brief. October 2021.
- Stone EC, Hofmeister M, Okasako-Schmucker DL, et al. Brief Summary of Findings on the Association Between Underlying Liver Diseases and Severe COVID-19 Outcomes. [print only, 1462K, 111 pages]CDC COVID-19 Scientific Brief. October 2021.
- So CN, Ryerson AB, Yeargin-Allsopp M, Kristie EN et al. Brief Summary of Findings on the Association Between Disabilities and Severe COVID-19 Outcomes. [1984K, 165 pages]CDC COVID-19 Scientific Brief.
- Okasako-Schmucker DL, Weissman D, Mazurek J et al. Brief Summary of Findings on the Association Between Interstitial Lung Diseases and Severe COVID-19 Outcomes. [print only, 837K, 67 pages]CDC COVID-19 Scientific Brief. October 2021.
- Hill AL, Whitfield G, Morford M et al. Brief Summary of Findings on the Association Between Physical Inactivity and Severe COVID-19 Outcomes. [931 KB, 63 pages] CDC COVID-19 Scientific Brief.
- Morford M, Green RF, Drzymalla E et al. Brief Summary of Findings on the Association Between Underlying Primary Immunodeficiency and Severe COVID-19 Outcomes. [print only, 705K, 41 pages] CDC COVID-19 Scientific Brief.
- Wassef M, Weissman D, Mazurek J et al. Brief Summary of Findings on the Association Between a History of Pulmonary Embolism or Pulmonary Hypertension and Severe COVID-19 Outcomes. [print only, 506K, 16 pages]CDC COVID-19 Scientific Brief. October 2021.
- Kumasaka JK, Jereb JA, Stone E et al. Brief Summary of Findings on the Association Between Tuberculosis and Severe COVID-19 Outcomes. [print only, 443K, 17 pages]CDC COVID-19 Scientific Brief. October 2021.
- Morford M, Weissman, D, Mazurek J, et al. Brief Summary of Findings on the Association Between Alpha-1 Antitrypsin Deficiency and Severe COVID-19 Outcomes. [print only, 533K, 27 pages]CDC COVID-19 Scientific Brief. October 2021.
- Henry MC, Weissman D, Mazurek J, et al.Brief Summary of Findings on the Association Between Underlying Bronchopulmonary Dysplasia (BPD) and Severe COVID-19 Outcomes. [print only, 375K, 12 pages]CDC COVID-19 Scientific Brief. October 2021.
- Okasako-Schmucker DL, Cornwell C, Mirabelli M, et al. Brief Summary of Findings on the Association Between Asthma and Severe COVID-19 Outcomes. [print only, 2,000KB, 142 pages] CDC COVID-19 Scientific Brief. June 2022.
- Kumasaka JK, Weissman D, Mazurek J, et al. Brief Summary of Findings on the Association Between COPD and Severe COVID-19 Outcomes. [print only, 2,000KB, 176 pages]CDC COVID-19 Scientific Brief. June 2022.
- So CN, Green RF, Drzymalia E, et al. Brief Summary of Findings on the Association Between Cystic Fibrosis and Severe COVID-19 Outcomes. [print only, 788K, 54 pages] CDC COVID-19 Scientific Brief. June 2022.
- Hill AL, Payne AB, Schieve LA, et al. Brief Summary of Findings on the Association Between Thalassemia and Severe COVID-19 Outcomes. [print only, 619 KB, 26 pages] CDC COVID-19 Scientific Brief. June 2022.
- Morford M, Brief Summary of Findings on the Association Between Hematologic Malignancy and Hematopoietic Stem Cell Transplant/Hematopoietic Cell Transplant and Severe COVID-19 Outcomes [886KB – 78 pages] CDC COVID-19 Clinical Care. January 2023.
- Supplement: Morford M, Koumans E, Giovanni J, et al. Brief Summary of Findings on the Association Between Secondary Immunosuppression from B-Cell-Depleting Therapy and Severe COVID-19 Outcomes [482 KB – 28 pages] CDC COVID-19 Clinical Care. January 2023.
See All References
- Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk Factors Associated With In-Hospital Mortality in a US National Sample of Patients With COVID-19. JAMA Network Open. 2020;3(12):e2029058-e2029058. doi:10.1001/jamanetworkopen.2020.29058
- De Giorgi A, Fabbian F, Greco S, et al. Prediction of in-hospital mortality of patients with SARS-CoV-2 infection by comorbidity indexes: an Italian internal medicine single center study. Eur Rev Med Pharmacol Sci. Oct 2020;24(19):10258-10266. doi:10.26355/eurrev_202010_23250
- Dominguez-Ramirez L, Rodriguez-Perez F, Sosa-Jurado F, Santos-Lopez G, Cortes-Hernandez P. The role of metabolic comorbidity in COVID-19 mortality of middle-aged adults. The case of Mexico. 2020:2020.12.15.20244160. doi:10.1101/2020.12.15.20244160 %J medRxiv
- Ahmad FB, Cisewski JA, Minino A, Anderson RN. Provisional Mortality Data – United States, 2020. MMWR Morb Mortal Wkly Rep. Apr 9 2021;70(14):519-522. doi:10.15585/mmwr.mm7014e1
- Prevention CDC. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#demographics
- Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of US Nursing Homes with COVID-19 Cases. Journal of the American Geriatrics Society. 2020. doi:10.1111/jgs.1661
- Grabowski DC, Mor V. Nursing Home Care in Crisis in the Wake of COVID-19. Journal of the American Medical Association. 2020. doi:10.1001/jama.2020.8524
- Brown KA, Jones A, Daneman N, et al. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. Journal of the American Medical Association, Internal Medicine. 2020. doi:10.1001/jamainternmed.2020.6466
- Sarah HY, See I, Kent AG, et al. Characterization of COVID-19 in assisted living facilities—39 states, October 2020. Morbidity and Mortality Weekly Report. 2020;69(46):1730.
- Fisman DN, Bogoch I, Lapointe-Shaw L, McCready J, Tuite AR. Risk factors associated with mortality among residents with coronavirus disease 2019 (COVID-19) in long-term care facilities in Ontario, Canada. Journal of the American Medical Association. 2020;3(7):e2015957-e2015957. doi:10.1001/jamanetworkopen.2020.15957
- Ko JY, Danielson ML, Town M, et al. Risk Factors for Coronavirus Disease 2019 (COVID-19)–Associated Hospitalization: COVID-19–Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clinical Infectious Diseases. 2021;72(11):e695-e703. doi:10.1093/cid/ciaa1419
- Wortham JM, Lee JT, Althomsons S, et al. Characteristics of Persons Who Died with COVID-19 – United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. Jul 17 2020;69(28):923-929. doi:10.15585/mmwr.mm6928e1
- Yang X, Zhang J, Chen S, et al. Demographic Disparities in Clinical Outcomes of COVID-19: Data From a Statewide Cohort in South Carolina. Open Forum Infect Dis. Sep 2021;8(9):ofab428. doi:10.1093/ofid/ofab428
- Rader B.; Gertz AL, D.; Gilmer, M.; Wronski, L.; Astley, C.; Sewalk, K.; Varrelman, T.; Cohen, J.; Parikh, R.; Reese, H.; Reed, C.; Brownstein J. Use of At-Home COVID-19 Tests — United States, August 23, 2021–March 12, 2022. MMWR Morb Mortal Wkly Rep. April 1, 2022;71(13):489–494. doi:10.15585/mmwr.mm7113e1
- Pingali C, Meghani M, Razzaghi H, et al. COVID-19 Vaccination Coverage Among Insured Persons Aged >/=16 Years, by Race/Ethnicity and Other Selected Characteristics – Eight Integrated Health Care Organizations, United States, December 14, 2020-May 15, 2021. MMWR Morb Mortal Wkly Rep. Jul 16 2021;70(28):985-990. doi:10.15585/mmwr.mm7028a1
- Wiltz JL, Feehan AK, Molinari NM, et al. Racial and Ethnic Disparities in Receipt of Medications for Treatment of COVID-19 – United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. Jan 21 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
- Prevention CDC. Health Disparities: Race and Hispanic Origin. Provisional Death Counts for Coronavirus Disease 2019. February 9, 2022.
- Saini KS, Tagliamento M, Lambertini M, et al. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur J Cancer. Nov 2020;139:43-50. doi:10.1016/j.ejca.2020.08.011
- Zhou Y, Yang Q, Chi J, et al. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. Oct 2020;99:47-56. doi:10.1016/j.ijid.2020.07.029
- Venkatesulu BP, Chandrasekar VT, Girdhar P, et al. A Systematic Review and Meta-Analysis of Cancer Patients Affected by a Novel Coronavirus. JNCI Cancer Spectrum. 2021;5(2) doi:10.1093/jncics/pkaa102
- Salunke AA, Nandy K, Pathak SK, et al. Impact of COVID -19 in cancer patients on severity of disease and fatal outcomes: A systematic review and meta-analysis. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2020/09/01/ 2020;14(5):1431-1437. doi:10.1016/j.dsx.2020.07.037
- Gao Y, Liu M, Shi S, et al. Cancer is associated with the severity and mortality of patients with COVID-19: a systematic review and meta-analysis. medRxiv. 2020:2020.05.01.20087031. doi:10.1101/2020.05.01.20087031
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology. Mar 2020;21(3):335-337. doi:10.1016/s1470-2045(20)30096-6
- Nepogodiev D, Bhangu A, Glasbey JC, et al. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. The Lancet. 2020;396(10243):27-38. doi:10.1016/S0140-6736(20)31182-X
- Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. Jun 20 2020;395(10241):1919-1926. doi:10.1016/S0140-6736(20)31173-9
- Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nature medicine. Aug 2020;26(8):1218-1223. doi:10.1038/s41591-020-0979-0
- Zhang H, Wang L, Chen Y, et al. Outcomes of novel coronavirus disease 2019 (COVID-19) infection in 107 patients with cancer from Wuhan, China. Cancer. Sep 1 2020;126(17):4023-4031. doi:10.1002/cncr.33042
- Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. Jun 20 2020;395(10241):1907-1918. doi:10.1016/S0140-6736(20)31187-9
- Wang Q, Berger NA, Xu R. Analyses of Risk, Racial Disparity, and Outcomes Among US Patients With Cancer and COVID-19 Infection. JAMA oncology. Dec 10 2020. doi:10.1001/jamaoncol.2020.6178
- Pranata R, Huang I, Lim MA, Wahjoepramono EJ, July J. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. Aug 2020;29(8):104949. doi:10.1016/j.jstrokecerebrovasdis.2020.104949
- Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. Apr 8 2020;12(7):6049-6057. doi:10.18632/aging.103000
- Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020;15(8):e0238215. doi:10.1371/journal.pone.0238215
- Khan MMA, Khan MN, Mustagir MG, Rana J, Islam MS, Kabir MI. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. Journal of global health. Dec 2020;10(2):020503. doi:10.7189/jogh.10.020503
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. European journal of internal medicine. Jun 2020;76:97-99. doi:10.1016/j.ejim.2020.04.043
- Chen R, Liang W, Jiang M, et al. Risk Factors of Fatal Outcome in Hospitalized Subjects With Coronavirus Disease 2019 From a Nationwide Analysis in China. Chest. Jul 2020;158(1):97-105. doi:10.1016/j.chest.2020.04.010
- Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. Aug 2020;584(7821):430-436. doi:10.1038/s41586-020-2521-4
- Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. The Journal of infection. Jun 2020;80(6):639-645. doi:10.1016/j.jinf.2020.03.019
- Pilgram L, Eberwein L, Wille K, et al. Clinical course and predictive risk factors for fatal outcome of SARS-CoV-2 infection in patients with chronic kidney disease. Infection. Aug 2021;49(4):725-737. doi:10.1007/s15010-021-01597-7
- Kang SH, Kim SW, Kim AY, Cho KH, Park JW, Do JY. Association between Chronic Kidney Disease or Acute Kidney Injury and Clinical Outcomes in COVID-19 Patients. J Korean Med Sci. Dec 28 2020;35(50):e434. doi:10.3346/jkms.2020.35.e434
- Fajgenbaum DC, Khor JS, Gorzewski A, et al. Treatments Administered to the First 9152 Reported Cases of COVID-19: A Systematic Review. Infect Dis Ther. Sep 2020;9(3):435-449. doi:10.1007/s40121-020-00303-8
- Gottlieb M, Sansom S, Frankenberger C, Ward E, Hota B. Clinical Course and Factors Associated With Hospitalization and Critical Illness Among COVID-19 Patients in Chicago, Illinois. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. Oct 2020;27(10):963-973. doi:10.1111/acem.14104
- Fernandes DM, Oliveira CR, Guerguis S, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. The Journal of pediatrics. Nov 14 2020; doi:10.1016/j.jpeds.2020.11.016
- Hernandez-Galdamez DR, Gonzalez-Block MA, Romo-Duenas DK, et al. Increased Risk of Hospitalization and Death in Patients with COVID-19 and Pre-existing Noncommunicable Diseases and Modifiable Risk Factors in Mexico. Archives of Medical Research. 2020;doi: do10.1016/j.arcmed.2020.07.003
- Menezes Soares RDC, Mattos LR, Raposo LM. Risk Factors for Hospitalization and Mortality due to COVID-19 in Espirito Santo State, Brazil. American Journal of Tropical Medicine and Hygiene. 2020;103(3):1184-1190. doi:10.4269/ajtmh.20-0483
- Oetjens MT, Luo JZ, Chang A, et al. Electronic health record analysis identifies kidney disease as the leading risk factor for hospitalization in confirmed COVID-19 patients. PloS one. 2020;15(11):e0242182. doi:10.1371/journal.pone.0242182
- Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. May 22 2020;369:m1966. doi:10.1136/bmj.m1966
- Reilev M, Kristensen KB, Pottegard A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. International journal of epidemiology. 2020. doi:10.1093/ije/dyaa140
- Suleyman G, Fadel RA, Malette KM, et al. Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit. JAMA Netw Open. Jun 1 2020;3(6):e2012270. doi:10.1001/jamanetworkopen.2020.12270
- Rastad H, Ejtahed HS, Mahdavi-Ghorabi A, et al. Factors associated with the poor outcomes in diabetic patients with COVID-19. Journal of Diabetes and Metabolic Disorders. 2020;doi: doi:0.1007/s40200-020-00646-6
- Fried MW, Crawford JM, Mospan AR, et al. Patient Characteristics and Outcomes of 11,721 Patients with COVID19 Hospitalized Across the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. doi:10.1093/cid/ciaa1268
- Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. Oct 2020;17(10):e1003406. doi:10.1371/journal.pmed.1003406
- Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute Kidney Injury in a National Cohort of Hospitalized US Veterans with COVID-19. Clin J Am Soc Nephrol. Nov 16 2020. doi:10.2215/CJN.09610620
- McKeigue PM, Weir A, Bishop J, et al. Rapid Epidemiological Analysis of Comorbidities and Treatments as risk factors for COVID-19 in Scotland (REACT-SCOT): A population-based case-control study. PLoS medicine. 2020;17(10):e1003374. doi:10.1371/journal.pmed.1003374
- Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. Aug 29 2020. doi:10.1093/cid/ciaa1198
- Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Annals of Epidemiology. 2020; doi:10.1016/j.annepidem.2020.08.005
- Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney international. 2020. doi:10.1016/j.kint.2020.07.030
- Omrani AS, Almaslamani MA, Daghfal J, et al. The first consecutive 5000 patients with Coronavirus Disease 2019 from Qatar; a nation-wide cohort study. BMC Infectious Diseases. 2020;20(1):777. doi:10.1186/s12879-020-05511-8
- Iaccarino G, Borghi C, Carugo S, et al. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the italian society of hypertension. PLoS ONE. 2020;15(10 October):e0237297. doi:10.1371/journal.pone.0237297
- Gu T, Chu Q, Yu Z, et al. History of coronary heart disease increased the mortality rate of patients with COVID-19: a nested case-control study. BMJ open. Sep 17 2020;10(9):e038976. doi:10.1136/bmjopen-2020-038976
- Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. Jama. Jun 2 2020;323(21):2195-2198. doi:10.1001/jama.2020.7202
- Hirsch JS, Ng JH, Ross DW, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. Jul 2020;98(1):209-218. doi:10.1016/j.kint.2020.05.006
- Gold JAW, Wong KK, Szablewski CM, et al. Characteristics and Clinical Outcomes of Adult Patients Hospitalized with COVID-19 – Georgia, March 2020. MMWR Morb Mortal Wkly Rep. May 8 2020;69(18):545-550. doi:10.15585/mmwr.mm6918e1
- Jering KS, Claggett BL, Cunningham JW, et al. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern Med. Jan 15 2021. doi:10.1001/jamainternmed.2020.9241
- Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 – COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. Apr 17 2020;69(15):458-464. doi:10.15585/mmwr.mm6915e3
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775 %J JAMA
- Lee JY, Hong SW, Hyun M, et al. Epidemiological and clinical characteristics of coronavirus disease 2019 in Daegu, South Korea. Int J Infect Dis. Sep 2020;98:462-466. doi:10.1016/j.ijid.2020.07.017
- Fadini GP, Morieri ML, Boscari F, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. Oct 2020;168:108374. doi:10.1016/j.diabres.2020.108374
- Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. Oct 2020;8(10):813-822. doi:10.1016/s2213-8587(20)30272-2
- Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic's Impact in Type 1 and Type 2 Diabetes. Diabetes Care. Dec 2 2020. doi:10.2337/dc20-2260
- Duarte-Salles T, Vizcaya D, Pistillo A, et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID-19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. medRxiv. Oct 30 2020. doi:10.1101/2020.10.29.20222083
- Bode B, Garrett V, Messler J, et al. Glycemic Characteristics and Clinical Outcomes of COVID-19 Patients Hospitalized in the United States. Journal of diabetes science and technology. Jul 2020;14(4):813-821. doi:10.1177/1932296820924469
- Vangoitsenhoven R, Martens P-J, van Nes F, et al. No Evidence of Increased Hospitalization Rate for COVID-19 in Community-Dwelling Patients With Type 1 Diabetes. 2020;43(10):e118-e119. doi:10.2337/dc20-1246 %J Diabetes Care
- Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes. Nov 17 2020. doi:10.1111/pedi.13158
- Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. Journal of endocrinological investigation. Jun 2020;43(6):867-869. doi:10.1007/s40618-020-01236-2
- Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. Sep 1 2020;370:m3320. doi:10.1136/bmj.m3320
- Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Cmaj. Apr 19 2021;193(16):E540-e548. doi:10.1503/cmaj.202604
- Zhu L, She ZG, Cheng X, et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. Jun 2 2020;31(6):1068-1077.e3. doi:10.1016/j.cmet.2020.04.021
- Chen Y, Yang D, Cheng B, et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care. Jul 2020;43(7):1399-1407. doi:10.2337/dc20-0660
- Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. Nov 27 2020. doi:10.1111/dom.14269
- de Almeida-Pititto B, Dualib PM, Zajdenverg L, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. doi:10.1186/s13098-020-00586-4
- Kow CS, Hasan SS. Mortality risk with preadmission metformin use in patients with COVID-19 and diabetes: A meta-analysis. J Med Virol. Sep 9 2020. doi:10.1002/jmv.26498
- Perez-Belmonte LM, Torres-Pena JD, Lopez-Carmona MD, et al. Mortality and other adverse outcomes in patients with type 2 diabetes mellitus admitted for COVID-19 in association with glucose-lowering drugs: a nationwide cohort study. BMC Med. Nov 16 2020;18(1):359. doi:10.1186/s12916-020-01832-2
- Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. The Journal of infection. Aug 2020;81(2):e16-e25. doi:10.1016/j.jinf.2020.04.021
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. May 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
- Del Sole F, Farcomeni A, Loffredo L, et al. Features of severe COVID-19: A systematic review and meta-analysis. European journal of clinical investigation. 2020;50(10):e13378. doi:10.1111/eci.13378
- Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Scientific Reports. 2021/03/18 2021;11(1):6283. doi:10.1038/s41598-021-85359-3
- Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. Dec 11 2020. doi:10.1016/s2352-3018(20)30305-2
- Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS (London, England). 2020;34(13):F3-F8. doi:10.1097/qad.0000000000002666
- Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV medicine. 2021;22(1):e1-e2. doi:10.1111/hiv.12920
- Härter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. Oct 2020;48(5):681-686. doi:10.1007/s15010-020-01438-z
- Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS-CoV-2 coinfected patients in Istanbul, Turkey. J Med Virol. Nov 2020;92(11):2288-2290. doi:10.1002/jmv.25955
- Ho H-e, Peluso MJ, Margus C, et al. Clinical Outcomes and Immunologic Characteristics of Coronavirus Disease 2019 in People With Human Immunodeficiency Virus. The Journal of Infectious Diseases. 2020;223(3):403-408. doi:10.1093/infdis/jiaa380
- Fond G, Nemani K, Etchecopar-Etchart D, et al. Association Between Mental Health Disorders and Mortality Among Patients With COVID-19 in 7 Countries: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2021. doi:10.1001/jamapsychiatry.2021.2274
- Ceban F, Nogo D, Carvalho IP, et al. Association Between Mood Disorders and Risk of COVID-19 Infection, Hospitalization, and Death: A Systematic Review and Meta-analysis. JAMA Psychiatry. Oct 1 2021;78(10):1079-1091. doi:10.1001/jamapsychiatry.2021.1818
- Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. Jul 14 2020;95(2):77-84. doi:10.1212/wnl.0000000000009673
- Zuin M, Guasti P, Roncon L, Cervellati C, Zuliani G. Dementia and the risk of death in elderly patients with COVID-19 infection: Systematic review and meta-analysis. International Journal of Geriatric Psychiatry. 2021;36(5):697-703. doi:10.1002/gps.5468
- Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The Impact of Dementia on the Clinical Outcome of COVID-19: A Systematic Review and Meta-Analysis. Journal of Alzheimer's Disease. 2020;78:1775-1782. doi:10.3233/JAD-201016
- Saragih ID, Saragih IS, Batubara SO, Lin C-J. Dementia as a mortality predictor among older adults with COVID-19: A systematic review and meta-analysis of observational study. Geriatric Nursing. 2021/09/01/ 2021;42(5):1230-1239. doi:10.1016/j.gerinurse.2021.03.007
- Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. Sep 1 2020;174(9):868-873. doi:10.1001/jamapediatrics.2020.1948
- Parri N, Lenge M, Buonsenso D. Children with Covid-19 in Pediatric Emergency Departments in Italy. New England Journal of Medicine. 2020;383(2):187-190. doi:10.1056/NEJMc2007617
- Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. Jun 30 2020. doi:10.1002/jmv.26237
- Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis. Nov 20 2020;103:246-256. doi:10.1016/j.ijid.2020.11.163
- Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: A systematic review and meta-analysis. Obesity Reviews. 2020;21(10):e13095. doi:10.1111/obr.13095
- Lighter J, Phillips M, Hochman S, et al. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis. Jul 28 2020;71(15):896-897. doi:10.1093/cid/ciaa415
- Tartof SY, Qian L, Hong V, et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization. Ann Intern Med. Nov 17 2020;173(10):773-781. doi:10.7326/m20-3742
- Hur K, Price CPE, Gray EL, et al. Factors Associated With Intubation and Prolonged Intubation in Hospitalized Patients With COVID-19. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. Jul 2020;163(1):170-178. doi:10.1177/0194599820929640
- Simonnet A, Chetboun M, Poissy J, et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity (Silver Spring). Jul 2020;28(7):1195-1199. doi:10.1002/oby.22831
- Aziz F, Mandelbrot D, Singh T, et al. Early Report on Published Outcomes in Kidney Transplant Recipients Compared to Nontransplant Patients Infected With Coronavirus Disease 2019. Transplantation proceedings. Nov 2020;52(9):2659-2662. doi:10.1016/j.transproceed.2020.07.002
- Ko JY, Danielson ML, Town M, et al. Risk Factors for COVID-19-associated hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System. Clin Infect Dis. Sep 18 2020. doi:10.1093/cid/ciaa1419
- Nakeshbandi M, Maini R, Daniel P, et al. The impact of obesity on COVID-19 complications: a retrospective cohort study. International journal of obesity (2005). Sep 2020;44(9):1832-1837. doi:10.1038/s41366-020-0648-x
- Hamer M, Gale CR, Kivimaki M, Batty GD. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci U S A. Sep 1 2020;117(35):21011-21013. doi:10.1073/pnas.2011086117
- Palaiodimos L, Kokkinidis DG, Li W, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism: clinical and experimental. Jul 2020;108:154262. doi:10.1016/j.metabol.2020.154262
- Di Martino D, Chiaffarino F, Patanè L, et al. Assessing risk factors for severe forms of COVID-19 in a pregnant population: A clinical series from Lombardy, Italy. International Journal of Gynecology & Obstetrics. 2021;152(2):275-277. doi:10.1002/ijgo.13435
- Khoury R, Bernstein PS, Debolt C, et al. Characteristics and Outcomes of 241 Births to Women With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection at Five New York City Medical Centers. Obstet Gynecol. Aug 2020;136(2):273-282. doi:10.1097/AOG.0000000000004025
- Metz TD, Clifton RG, Hughes BL, et al. Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. Apr 1 2021;137(4):571-580. doi:10.1097/AOG.0000000000004339
- Galang RR, Newton SM, Woodworth KR, et al. Risk factors for illness severity among pregnant women with confirmed SARS-CoV-2 infection – Surveillance for Emerging Threats to Mothers and Babies Network, 20 state, local, and territorial health departments, March 29, 2020 -January 8, 2021. medRxiv. 2021:2021.02.27.21252169. doi:10.1101/2021.02.27.21252169
- Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. The Journal of Maternal-Fetal & Neonatal Medicine. 2022/04/18 2022;35(8):1619-1622. doi:10.1080/14767058.2020.1759541
- Collin J, Bystrom E, Carnahan A, Ahrne M. Public Health Agency of Sweden's Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet Gynecol Scand. Jul 2020;99(7):819-822. doi:10.1111/aogs.13901
- Li N, Han L, Peng M, et al. Maternal and Neonatal Outcomes of Pregnant Women With Coronavirus Disease 2019 (COVID-19) Pneumonia: A Case-Control Study. Clin Infect Dis. Nov 19 2020;71(16):2035-2041. doi:10.1093/cid/ciaa352
- Chen L, Li Q, Zheng D, et al. Clinical Characteristics of Pregnant Women with Covid-19 in Wuhan, China. New England Journal of Medicine. 2020;382(25):e100. doi:10.1056/NEJMc2009226
- Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. American Journal of Obstetrics & Gynecology MFM. 2020/05/01/ 2020;2(2, Supplement):100118. doi:10.1016/j.ajogmf.2020.100118
- Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. American Journal of Obstetrics and Gynecology. 2020/12/01/ 2020;223(6):911.e1-911.e14. doi:10.1016/j.ajog.2020.05.031
- Pierce-Williams RAM, Burd J, Felder L, et al. Clinical course of severe and critical coronavirus disease 2019 in hospitalized pregnancies: a United States cohort study. Am J Obstet Gynecol MFM. Aug 2020;2(3):100134. doi:10.1016/j.ajogmf.2020.100134
- Savasi VM, Parisi F, Patane L, et al. Clinical Findings and Disease Severity in Hospitalized Pregnant Women With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. Aug 2020;136(2):252-258. doi:10.1097/AOG.0000000000003979
- Ellington S, Strid P, Tong VT, et al. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status – United States, January 22-June 7, 2020. MMWR Morb Mortal Wkly Rep. Jun 26 2020;69(25):769-775. doi:10.15585/mmwr.mm6925a1
- Zambrano LD, Ellington S, Strid P, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status – United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. Nov 6 2020;69(44):1641-1647. doi:10.15585/mmwr.mm6944e3
- Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. Jun 2020;167:105941. doi:10.1016/j.rmed.2020.105941
- Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. Aug 24 2020;22(9):1653-1656. doi:10.1093/ntr/ntaa082
- Guo FR. Active smoking is associated with severity of coronavirus disease 2019 (COVID-19): An update of a meta-analysis. Tobacco induced diseases. 2020;18:37. doi:10.18332/tid/121915
- Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. Oct 2020;92(10):1915-1921. doi:10.1002/jmv.25889
- Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). European journal of internal medicine. May 2020;75:107-108. doi:10.1016/j.ejim.2020.03.014
- Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PloS one. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147
- Li J, He X, Yuan Y, et al. Meta-analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia. American journal of infection control. Jan 2021;49(1):82-89. doi:10.1016/j.ajic.2020.06.008
- Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Therapeutic advances in chronic disease. 2020;11:2040622320935765. doi:10.1177/2040622320935765
- Sanchez-Ramirez DC, Mackey D. Underlying respiratory diseases, specifically COPD, and smoking are associated with severe COVID-19 outcomes: A systematic review and meta-analysis. Respir Med. Sep 2020;171:106096. doi:10.1016/j.rmed.2020.106096
- Akalin E, Azzi Y, Bartash R, et al. Covid-19 and Kidney Transplantation. N Engl J Med. Jun 18 2020;382(25):2475-2477. doi:10.1056/NEJMc2011117
- Ketcham SW, Adie SK, Malliett A, et al. Coronavirus Disease-2019 in Heart Transplant Recipients in Southeastern Michigan: A Case Series. J Card Fail. Jun 2020;26(6):457-461. doi:10.1016/j.cardfail.2020.05.008
- Latif F, Farr MA, Clerkin KJ, et al. Characteristics and Outcomes of Recipients of Heart Transplant With Coronavirus Disease 2019. JAMA cardiology. Oct 1 2020;5(10):1165-1169. doi:10.1001/jamacardio.2020.2159
- Zhu L, Xu X, Ma K, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. Jul 2020;20(7):1859-1863. doi:10.1111/ajt.15869
- Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. American Journal of Transplantation. 2020;20(7):1849-1858. doi:10.1111/ajt.15929
- Travi G, Rossotti R, Merli M, et al. Clinical outcome in solid organ transplant recipients with COVID-19: A single-center experience. Am J Transplant. Sep 2020;20(9):2628-2629. doi:10.1111/ajt.16069
- Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. Oct 2020;20(10):2876-2882. doi:10.1111/ajt.16062
- Yi SG, Rogers AW, Saharia A, et al. Early Experience With COVID-19 and Solid Organ Transplantation at a US High-volume Transplant Center. Transplantation. 2020;104(11):2208-2214. doi:10.1097/TP.0000000000003339
- Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID-19: A case series from the United States. American Journal of Transplantation. 2020;20(11):3225-3233. doi:10.1111/ajt.16079
- Hoek RAS, Manintveld OC, Betjes MGH, et al. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33(9):1099-1105. doi:10.1111/tri.13662
- Iacovoni A, Boffini M, Pidello S, et al. A case series of novel coronavirus infection in heart transplantation from 2 centers in the pandemic area in the North of Italy. J Heart Lung Transplant. 2020;39(10):1081-1088. doi:10.1016/j.healun.2020.06.016
- Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800-1808. doi:10.1111/ajt.15941
- Kates OS, Haydel BM, Florman SS, et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. Dec 6 2021;73(11):e4090-e4099. doi:10.1093/cid/ciaa1097
- Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. The Lancet Haematology. Mar 2021;8(3):e185-e193. doi:10.1016/s2352-3026(20)30429-4
- Ljungman P, de la Camara R, Mikulska M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021/10/01 2021;35(10):2885-2894. doi:10.1038/s41375-021-01302-5
- Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: A large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. Jan 2022;36(1):e14492. doi:10.1111/ctr.14492
- Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. Dec 2020;141:92-104. doi:10.1016/j.ejca.2020.09.028
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. Aug 2020;159(2):481-491.e3. doi:10.1053/j.gastro.2020.05.032
- Michelena X, Borrell H, López-Corbeto M, et al. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Seminars in arthritis and rheumatism. Aug 2020;50(4):564-570. doi:10.1016/j.semarthrit.2020.05.001
- Di Giorgio A, Nicastro E, Speziani C, et al. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. Journal of hepatology. Sep 2020;73(3):702-705. doi:10.1016/j.jhep.2020.05.008
- Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID-19 in children on immunosuppressive medication. The Lancet Child & adolescent health. Jul 2020;4(7):e17-e18. doi:10.1016/s2352-4642(20)30145-0
- Montero-Escribano P, Matías-Guiu J, Gómez-Iglesias P, Porta-Etessam J, Pytel V, Matias-Guiu JA. Anti-CD20 and COVID-19 in multiple sclerosis and related disorders: A case series of 60 patients from Madrid, Spain. Multiple sclerosis and related disorders. Jul 2020;42:102185. doi:10.1016/j.msard.2020.102185
- Alsaied T, Aboulhosn JA, Cotts TB, et al. Coronavirus Disease 2019 (COVID-19) Pandemic Implications in Pediatric and Adult Congenital Heart Disease. J Am Heart Assoc. Jun 16 2020;9(12):e017224. doi:10.1161/jaha.120.017224
- Sanna G, Serrau G, Bassareo PP, Neroni P, Fanos V, Marcialis MA. Children's heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur J Pediatr. Jul 2020;179(7):1079-1087. doi:10.1007/s00431-020-03699-0
- Sabatino J, Ferrero P, Chessa M, et al. COVID-19 and Congenital Heart Disease: Results from a Nationwide Survey. J Clin Med. Jun 8 2020;9(6). doi:10.3390/jcm9061774
- Bellino S, Punzo O, Rota MC, et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics. Oct 2020;146(4). doi:10.1542/peds.2020-009399
- DeBiasi RL, Song X, Delaney M, et al. Severe Coronavirus Disease-2019 in Children and Young Adults in the Washington, DC, Metropolitan Region. The Journal of pediatrics. Aug 2020;223:199-203.e1. doi:10.1016/j.jpeds.2020.05.007
- Chao JY, Derespina KR, Herold BC, et al. Clinical Characteristics and Outcomes of Hospitalized and Critically Ill Children and Adolescents with Coronavirus Disease 2019 at a Tertiary Care Medical Center in New York City. The Journal of pediatrics. Aug 2020;223:14-19.e2. doi:10.1016/j.jpeds.2020.05.006
- Kim DW, Byeon KH, Kim J, Cho KD, Lee N. The Correlation of Comorbidities on the Mortality in Patients with COVID-19: an Observational Study Based on the Korean National Health Insurance Big Data. J Korean Med Sci. Jul 6 2020;35(26):e243. doi:10.3346/jkms.2020.35.e243
- González-Dambrauskas S, Vásquez-Hoyos P, Camporesi A, et al. Pediatric Critical Care and COVID-19. Pediatrics. Sep 2020;146(3). doi:10.1542/peds.2020-1766
- Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. Sep 2020;4(9):653-661. doi:10.1016/s2352-4642(20)30177-2
- Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, Clinical Features, and Disease Severity in Patients With Coronavirus Disease 2019 (COVID-19) in a Children's Hospital in New York City, New York. JAMA Pediatr. Oct 1 2020;174(10):e202430. doi:10.1001/jamapediatrics.2020.2430
- Verma S, Lumba R, Dapul HM, et al. Characteristics of Hospitalized Children With SARS-CoV-2 in the New York City Metropolitan Area. Hosp Pediatr. Jan 2021;11(1):71-78. doi:10.1542/hpeds.2020-001917
- Leon-Abarca JA. Obesity and immunodeficiencies are the main pre-existing conditions associated with mild to moderate COVID-19 in children. Pediatr Obes. Dec 2020;15(12):e12713. doi:10.1111/ijpo.12713
- Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID-19 in children. Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. Jul 2020;27(5):235-238. doi:10.1016/j.arcped.2020.05.010
- Heilbronner C, Berteloot L, Tremolieres P, et al. Patients with sickle cell disease and suspected COVID-19 in a paediatric intensive care unit. British journal of haematology. 2020;190(1):e21-e24. doi:10.1111/bjh.16802
- Arlet J-B, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. The Lancet Haematology. 2020/09/01/ 2020;7(9):e632-e634. doi:10.1016/S2352-3026(20)30204-0
- Odièvre MH, de Marcellus C, Ducou Le Pointe H, et al. Dramatic improvement after tocilizumab of severe COVID-19 in a child with sickle cell disease and acute chest syndrome. American journal of hematology. Aug 2020;95(8):E192-e194. doi:10.1002/ajh.25855
- McCloskey KA, Meenan J, Hall R, Tsitsikas DA. COVID-19 infection and sickle cell disease: a UK centre experience. British journal of haematology. Jul 2020;190(2):e57-e58. doi:10.1111/bjh.16779
- Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19). American journal of hematology. Jun 2020;95(6):725-726. doi:10.1002/ajh.25821
- Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID-19 infection in patients with sickle cell disease. British journal of haematology. Jun 2020;189(5):851-852. doi:10.1111/bjh.16734
- Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus Disease among Persons with Sickle Cell Disease, United States, March 20-May 21, 2020. Emerg Infect Dis. Oct 2020;26(10):2473-2476. doi:10.3201/eid2610.202792
- Al-Hebshi A, Zolaly M, Alshengeti A, et al. A Saudi family with sickle cell disease presented with acute crises and COVID-19 infection. Pediatric blood & cancer. Sep 2020;67(9):e28547. doi:10.1002/pbc.28547
- Allison D, Campbell-Lee S, Crane J, et al. Red blood cell exchange to avoid intubating a COVID-19 positive patient with sickle cell disease? Journal of clinical apheresis. Aug 2020;35(4):378-381. doi:10.1002/jca.21809
- Appiah-Kubi A, Acharya S, Fein Levy C, et al. Varying presentations and favourable outcomes of COVID-19 infection in children and young adults with sickle cell disease: an additional case series with comparisons to published cases. British journal of haematology. Aug 2020;190(4):e221-e224. doi:10.1111/bjh.17013
- Azerad MA, Bayoudh F, Weber T, et al. Sickle cell disease and COVID-19: Atypical presentations and favorable outcomes. EJHaem. Aug 4 2020. doi:10.1002/jha2.74
- Chakravorty S, Padmore-Payne G, Ike F, et al. COVID-19 in patients with sickle cell disease – a case series from a UK Tertiary Hospital. Haematologica. Jun 11 2020;105(11). doi:10.3324/haematol.2020.254250
- De Luna G, Habibi A, Deux JF, et al. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. American journal of hematology. Jul 2020;95(7):876-878. doi:10.1002/ajh.25833
- Ershler WB, Holbrook ME. Sickle cell anemia and COVID-19: Use of voxelotor to avoid transfusion. Transfusion. Dec 2020;60(12):3066-3067. doi:10.1111/trf.16068
- Jacob S, Dworkin A, Romanos-Sirakis E. A pediatric patient with sickle cell disease presenting with severe anemia and splenic sequestration in the setting of COVID-19. Pediatric blood & cancer. Dec 2020;67(12):e28511. doi:10.1002/pbc.28511
- Justino CC, Campanharo FF, Augusto MN, Morais SC, Figueiredo MS. COVID-19 as a trigger of acute chest syndrome in a pregnant woman with sickle cell anemia. Hematology, transfusion and cell therapy. Jul-Sep 2020;42(3):212-214. doi:10.1016/j.htct.2020.06.003
- Morrone KA, Strumph K, Liszewski MJ, et al. Acute chest syndrome in the setting of SARS-COV-2 infections-A case series at an urban medical center in the Bronx. Pediatric blood & cancer. Nov 2020;67(11):e28579. doi:10.1002/pbc.28579
- Balanchivadze N, Kudirka AA, Askar S, et al. Impact of COVID-19 Infection on 24 Patients with Sickle Cell Disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin. Jul 2020;44(4):284-289. doi:10.1080/03630269.2020.1797775
- Allen B, El Shahawy O, Rogers ES, Hochman S, Khan MR, Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in New York City: January-October 2020. Journal of public health (Oxford, England). Dec 26 2020. doi:10.1093/pubmed/fdaa241
- Ji W, Huh K, Kang M, et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: a Nationwide Case-Control Study. J Korean Med Sci. Jun 29 2020;35(25):e237. doi:10.3346/jkms.2020.35.e237
- Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Molecular psychiatry. Sep 14 2020:1-10. doi:10.1038/s41380-020-00880-7
- Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. The lancet Psychiatry. Dec 2020;7(12):1025-1031. doi:10.1016/s2215-0366(20)30421-1
- Baillargeon J, Polychronopoulou E, Kuo YF, Raji MA. The Impact of Substance Use Disorder on COVID-19 Outcomes. Psychiatr Serv. Nov 3 2020:appips202000534. doi:10.1176/appi.ps.202000534
- Matsushita K, Ding N, Kou M, et al. The Relationship of COVID-19 Severity with Cardiovascular Disease and Its Traditional Risk Factors: A Systematic Review and Meta-Analysis. Global heart. Sep 22 2020;15(1):64. doi:10.5334/gh.814
- Wu T, Zuo Z, Kang S, et al. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging and disease. Jul 2020;11(4):874-894. doi:10.14336/ad.2020.0520
- Guo X, Zhu Y, Hong Y. Decreased Mortality of COVID-19 With Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients With Hypertension: A Meta-Analysis. Hypertension. Aug 2020;76(2):e13-e14. doi:10.1161/HYPERTENSIONAHA.120.15572
- Zhang J, Wu J, Sun X, et al. Association of hypertension with the severity and fatality of SARS-CoV-2 infection: A meta-analysis. Epidemiol Infect. May 28 2020;148:e106. doi:10.1017/S095026882000117X
- Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: A systematic review, meta-analysis and meta-regression. Journal of the renin-angiotensin-aldosterone system : JRAAS. Apr-Jun 2020;21(2):1470320320926899. doi:10.1177/1470320320926899
- Javanmardi F, Keshavarzi A, Akbari A, Emami A, Pirbonyeh N. Prevalence of underlying diseases in died cases of COVID-19: A systematic review and meta-analysis. PLoS One. 2020;15(10):e0241265. doi:10.1371/journal.pone.0241265
- Iaccarino G, Grassi G, Borghi C, et al. Age and Multimorbidity Predict Death Among COVID-19 Patients: Results of the SARS-RAS Study of the Italian Society of Hypertension. Hypertension. 08 2020;76(2):366-372. doi:10.1161/HYPERTENSIONAHA.120.15324
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. The European respiratory journal. May 2020;55(5). doi:10.1183/13993003.00547-2020
- Kim L, Garg S, O'Halloran A, et al. Risk Factors for Intensive Care Unit Admission and In-hospital Mortality among Hospitalized Adults Identified through the U.S. Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis. Jul 16 2020. doi:10.1093/cid/ciaa1012
- Ran J, Song Y, Zhuang Z, et al. Blood pressure control and adverse outcomes of COVID-19 infection in patients with concomitant hypertension in Wuhan, China. Hypertension research : official journal of the Japanese Society of Hypertension. Nov 2020;43(11):1267-1276. doi:10.1038/s41440-020-00541-w
- Yanover C, Mizrahi B, Kalkstein N, et al. What Factors Increase the Risk of Complications in SARS-CoV-2-Infected Patients? A Cohort Study in a Nationwide Israeli Health Organization. JMIR public health and surveillance. Aug 25 2020;6(3):e20872. doi:10.2196/20872
- Killerby ME, Link-Gelles R, Haight SC, et al. Characteristics Associated with Hospitalization Among Patients with COVID-19 – Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. Jun 26 2020;69(25):790-794. doi:10.15585/mmwr.mm6925e1
- Chen R, Yang J, Gao X, et al. Influence of blood pressure control and application of renin-angiotensin-aldosterone system inhibitors on the outcomes in COVID-19 patients with hypertension. J Clin Hypertens (Greenwich). Nov 2020;22(11):1974-1983. doi:10.1111/jch.14038
- Kompaniyets L, Pennington AF, Goodman AB, et al. Underlying Medical Conditions and Severe Illness Among 540,667 Adults Hospitalized With COVID-19, March 2020-March 2021. Preventing chronic disease. Jul 1 2021;18:E66. doi:10.5888/pcd18.210123
- Khoshnood R, Zali A, Tafreshinejad A, et al. Parkinson's disease and COVID-19: a systematic review and meta-analysis. November 2021. Neurological Sciences. doi:10.1007/s10072-021-05756-4
- Ufongene C, Van Hyfte G, Agarwal P, et al. Older adults with epilepsy and COVID-19: Outcomes in a multi-hospital health system. January 2024. Seizure: European Journal of Epilepsy. doi:10.1016/j.seizure.2023.11.018
- Sharathkumar A, Wendt L, Ortman C, et al. COVID-19 outcomes in persons with hemophilia: results from a US-based national COVID-19 surveillance registry. January 2024. Journal of Thrombosis and Haemostasis. doi:10.1016/j.jtha.2023.04.040
- Kurniawan A, Hariyanto, TI. Non-alcoholic fatty liver disease (NAFLD) and COVID-19 outcomes: A systematic review, meta-analysis, and meta regression. April 2023. Narra J. doi:10.52225/narra.v3i1.102
- * Indicates presence of evidence for pregnant and non-pregnant people
- ‡ Underlying conditions for which there is evidence in pediatric patients
- ^ Risk may be further increased for people receiving dialysis
