At a glance
This page includes recommendations for health care providers that address provision and use of progestin-only pills. This information comes from the 2024 U.S. Selected Practice Recommendations for Contraceptive Use (U.S. SPR).
Overview
Progestin-only pills (POPs) contain only a progestin and no estrogen. Three formulations are currently available in the United States: norethindrone, norgestrel, and drospirenone (DRSP). Approximately seven out of 100 POP users become pregnant in the first year with typical use.[28] POPs are reversible and can be used by patients of all ages. POPs do not protect against sexually transmitted infections (STIs), including human immunodeficiency virus (HIV) infection, and patients using POPs should be counseled that consistent and correct use of external (male) latex condoms reduces the risk for STIs, including HIV infection.[31] Use of internal (female) condoms can provide protection from STIs, including HIV infection, although data are limited.[31] Patients also should be counseled that pre-exposure prophylaxis (PrEP), when taken as prescribed, is highly effective for preventing HIV infection.[32]
Initiation of POPs
Timing
- All POPs may be started at any time if it is reasonably certain that the patient is not pregnant (Box 3).
Need for Back-Up Contraception
- Norethindrone or norgestrel POPs:
- If norethindrone or norgestrel POPs are started within the first 5 days since menstrual bleeding started, no additional contraceptive protection is needed.
- If norethindrone or norgestrel POPs are started >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- If norethindrone or norgestrel POPs are started within the first 5 days since menstrual bleeding started, no additional contraceptive protection is needed.
- DRSP POPs:
- If DRSP POPs are started on the first day of menstrual bleeding, no additional contraceptive protection is needed.
- If DRSP POPs are started >1 day since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days.
- If DRSP POPs are started on the first day of menstrual bleeding, no additional contraceptive protection is needed.
Special Considerations
Amenorrhea (Not Postpartum)
- Timing: All POPs may be started at any time if it is reasonably certain that the patient is not pregnant (Box 3).
- Need for back-up contraception:
- Norethindrone or norgestrel POPs: The patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- DRSP POPs: The patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days.
- Norethindrone or norgestrel POPs: The patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
Postpartum (Breastfeeding)
- Timing: All POPs may be started at any time, including immediately postpartum (U.S. MEC 2 if <30 days postpartum; U.S. MEC 1 if ≥30 days postpartum),[1] if it is reasonably certain that the patient is not pregnant (Box 3).
- Need for back-up contraception: If the patient is <6 months postpartum, amenorrheic, and fully or nearly fully breastfeeding (exclusively breastfeeding or the vast majority [≥85%] of feeds are breastfeeds),[44] no additional contraceptive protection is needed.
- Norethindrone or norgestrel POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days. If the patient’s menstrual cycle has returned and it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- DRSP POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days. If the patient’s menstrual cycle has returned and it has been >1 day since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days.
- Norethindrone or norgestrel POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days. If the patient’s menstrual cycle has returned and it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
Postpartum (Nonbreastfeeding)
- Timing: All POPs may be started at any time, including immediately postpartum (U.S. MEC 1),[1] if it is reasonably certain that the patient is not pregnant (Box 3).
- Need for back-up contraception: If the patient is <21 days postpartum, no additional contraceptive protection is needed.
- Norethindrone or norgestrel POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days. If the patient’s menstrual cycle has returned and it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- DRSP POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days. If the patient’s menstrual cycle has returned and it has been >1 day since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days.
- Norethindrone or norgestrel POPs: A patient who is ≥21 days postpartum and whose menstrual cycle has not returned needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days. If the patient’s menstrual cycle has returned and it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
Postabortion (Spontaneous or Induced)
- Timing: All POPs may be started at any time postabortion, including immediately after abortion completion, if it is reasonably certain that the patient is not pregnant (Box 3), or at the time of medication abortion initiation (U.S. MEC 1).[1]
- Need for back-up contraception: The patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days for norethindrone or norgestrel POPs or for the next 7 days for DRSP POPs, unless POPs are started at the time of an abortion.
Switching from Another Contraceptive Method
- Timing: All POPs may be started immediately if it is reasonably certain that the patient is not pregnant (Box 3). Waiting for the patient's next menstrual cycle is unnecessary.
- Need for back-up contraception:
- Norethindrone or norgestrel POPs: If it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- DRSP POPs: If it has been >1 day since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 7 days.
- Norethindrone or norgestrel POPs: If it has been >5 days since menstrual bleeding started, the patient needs to abstain from sexual intercourse or use barrier methods (e.g., condoms) for the next 2 days.
- Switching from an intrauterine device (IUD): In addition to the need for back-up contraception when starting POPs, there might be additional concerns when switching from an IUD. If the patient has had sexual intercourse since the start of their current menstrual cycle and it has been >5 days since menstrual bleeding started, theoretically, residual sperm might be in the genital tract, which could lead to fertilization if ovulation occurs. A health care provider may consider any of the following options to address the potential for residual sperm:
- Advise the patient to retain the IUD for at least 7 days after POPs are initiated and return for IUD removal.
- Advise the patient to abstain from sexual intercourse or use barrier methods (e.g., condoms) for 7 days before removing the IUD and switching to the new method. The patient should also follow the back-up contraception recommendations for either norethindrone or norgestrel POPs or for DRSP POPs.
- If the patient cannot return for IUD removal and has not abstained from sexual intercourse or used barrier methods (e.g., condoms) for 7 days, advise the patient to use ECPs at the time of IUD removal. All POPs may be started immediately after use of ECPs (with the exception of UPA). All POPs may be started no sooner than 5 days after use of UPA. The patient should also follow the back-up contraception recommendations for either norethindrone or norgestrel POPs or for DRSP POPs.
- Advise the patient to retain the IUD for at least 7 days after POPs are initiated and return for IUD removal.
Comments and Evidence Summary
In situations in which the health care provider is uncertain whether the patient might be pregnant, the benefits of starting POPs likely exceed any risk. Therefore, starting POPs should be considered at any time, with a follow-up pregnancy test in 2–4 weeks. (As appropriate, see recommendations for Emergency Contraception.)
Norethindrone or norgestrel POPs
Unlike COCs, which inhibit ovulation as the primary mechanism of action, norethindrone or norgestrel POPs inhibit ovulation in about half of cycles, although the rates vary widely by person.[333] Peak serum steroid levels are reached about 2 hours after administration, followed by rapid distribution and elimination, such that by 24 hours after administration, serum steroid levels are near baseline.[333] Therefore, taking norethindrone or norgestrel POPs at approximately the same time each day is important. An estimated 48 hours of norethindrone or norgestrel POP use has been deemed necessary to achieve the contraceptive effects on cervical mucus.[333] If a patient needs to use additional contraceptive protection when switching to norethindrone or norgestrel POPs from another contraceptive method, consider continuing their previous method for 2 days after starting norethindrone or norgestrel POPs. No direct evidence was found regarding the effects of starting norethindrone or norgestrel POPs at different times of the cycle.
DRSP POPs
DRSP POPs are more similar in mechanism of action to COCs, with inhibition of ovulation as the primary mechanism of action.[334] Therefore, the recommendations for starting and using a back-up method are similar to COC recommendations. If a patient needs to use additional contraceptive protection when switching to DRSP POPs from another contraceptive method, consider continuing their previous method for 7 days after starting DRSP POPs. No direct evidence was found regarding the effects of starting DRSP POPs at different times of the cycle.
Examinations and tests needed before initiation of POPs
Among healthy patients, no examinations or tests are needed before initiation of POPs, although a baseline weight and BMI measurement might be useful for addressing any concerns about changes in weight over time (Table 5). Patients with known medical problems or other special conditions might need additional examinations or tests before being determined to be appropriate candidates for a particular method of contraception. The U.S. MEC might be useful in such circumstances.[1]
Abbreviations: BMI = body mass index; STI = sexually transmitted infection; U.S. MEC = U.S. Medical Eligibility Criteria for Contraceptive Use.
* Class A: Essential and mandatory in all circumstances for safe and effective use of the contraceptive method.
Class B: Contributes substantially to safe and effective use, but implementation may be considered within the public health context, service context, or both; the risk of not performing an examination or test should be balanced against the benefits of making the contraceptive method available.
Class C: Does not contribute substantially to safe and effective use of the contraceptive method. (Source: World Health Organization. Selected practice recommendations for contraceptive use, 2nd ed. Geneva, Switzerland: WHO Press; 2004.)
† Weight (BMI) measurement is not needed to determine medical eligibility for any methods of contraception because all methods can be used (U.S. MEC 1) or generally can be used (U.S. MEC 2) among patients with obesity (BMI ≥30 kg/m2). However, measuring weight and calculating BMI at baseline might be helpful for discussing concerns about any changes in weight and whether changes might be related to use of the contraceptive method.
Weight (BMI): Patients with obesity (BMI ≥30 kg/m2) can use POPs (U.S. MEC 1);[1] therefore, screening for obesity is not necessary for the safe initiation of POPs. However, measuring weight and calculating BMI at baseline might be helpful for discussing concerns about any changes in weight and whether changes might be related to use of the contraceptive method.
Bimanual examination and cervical inspection: Pelvic examination is not necessary before initiation of POPs because it does not facilitate detection of conditions for which POPs would be unsafe. Although patients with certain conditions or characteristics should not use (U.S. MEC 4) or generally should not use (U.S. MEC 3) POPs,[1] none of these conditions are likely to be detected by pelvic examination.[172] A systematic review identified two case-control studies that compared delayed versus immediate pelvic examination before initiation of hormonal contraceptives, specifically oral contraceptives or DMPA.[23] No differences in risk factors for cervical neoplasia, incidence of STIs, incidence of abnormal Papanicolaou smears, or incidence of abnormal findings from wet mounts were observed (Level of evidence: II-2 fair, direct).
Lipids: Screening for dyslipidemias is not necessary for the safe initiation of POPs because of the low likelihood of clinically significant changes with use of hormonal contraceptives. A systematic review did not identify any evidence regarding outcomes among women who were screened versus not screened with lipid measurement before initiation of hormonal contraceptives.[24] During 2015–2016 among women aged 20–39 years in the United States, 6.7% had high cholesterol, defined as total serum cholesterol >240 mg/dL.[111] Studies have reported mixed results about the effects of hormonal methods on lipid levels among both healthy women and women with baseline lipid abnormalities, and the clinical significance of these changes is unclear.[112–115]
Liver enzymes: Although patients with hepatocellular carcinoma generally should not use POPs (U.S. MEC 3),[1] patients with benign liver tumors, viral hepatitis, or cirrhosis can use (U.S. MEC 1) or generally can use (U.S. MEC 2) POPs; screening for liver disease before initiation of POPs is not necessary because of the low prevalence of these conditions and the high likelihood that patients with liver disease already would have had the condition diagnosed. A systematic review did not identify any evidence regarding outcomes among women who were screened versus not screened with liver enzyme tests before initiation of hormonal contraceptives.[24] During 2012, among U.S. women, the percentage with liver disease (not further specified) was 1.3%.[116] During 2013, the incidence of acute hepatitis A, B, or C was ≤1 per 100,000 U.S. population.[117] During 2002–2011, the incidence of liver cancer among U.S. women was approximately 3.7 per 100,000 population.[118]
Clinical breast examination: Although patients with current breast cancer should not use POPs (U.S. MEC 4), screening asymptomatic patients with a clinical breast examination before initiating POPs is not necessary because of the low prevalence of breast cancer among women of reproductive age.[1] A systematic review did not identify any evidence regarding outcomes among women who were screened versus not screened with a clinical breast examination before initiation of hormonal contraceptives.[23] The incidence of breast cancer among women of reproductive age in the United States is low. During 2020, the incidence of breast cancer among women aged <50 years was approximately 45.9 per 100,000 women.[119]
Other screening: Patients with hypertension, diabetes, iron-deficiency anemia, thrombophilia, cervical intraepithelial neoplasia, cervical cancer, STIs, or HIV infection can use (U.S. MEC 1) or generally can use (U.S. MEC 2) POPs.[1] Therefore, screening for these conditions is not necessary for the safe initiation of POPs.
Number of pill packs that should be provided at initial and return visits
- At the initial and return visit, provide or prescribe up to a 1-year supply of POPs (e.g., 13 28-day pill packs), depending on the patient's preferences and anticipated use.
- A patient should be able to obtain POPs easily in the amount and at the time they need them.
Comments and Evidence Summary
The more pill packs provided up to 13 cycles, the higher the continuation rates. Restricting the number of pill packs distributed or prescribed can be a barrier for patients who want to continue POP use and might increase risk for pregnancy.
A systematic review of the evidence suggested that providing a greater number of pill packs was associated with increased continuation.[20] Studies that compared provision of one versus 12 packs, one versus 12 or 13 packs, or three versus seven packs found increased continuation of pill use among women provided with more pill packs.[287–289] However, one study found no difference in continuation when patients were provided one and then three packs versus four packs all at once.[290] In addition to continuation, a greater number of pill packs provided was associated with fewer pregnancy tests, fewer pregnancies, and lower cost per client. However, a greater number of pill packs (13 packs versus three packs) also was associated with increased pill wastage in one study[288] (Level of evidence: I to II-2, fair, direct).
Routine follow-up after POP initiation
These recommendations address when routine follow-up is recommended for safe and effective continued use of contraception for healthy patients. The recommendations refer to general situations and might vary for different users and different situations. Specific populations who might benefit from more frequent follow-up visits include adolescents, those with certain medical conditions or characteristics, and those with multiple medical conditions.
- Advise the patient that they may contact their provider at any time to discuss side effects or other problems or if they want to change the method being used. No routine follow-up visit is required.
- At other routine visits, health care providers seeing POP users should do the following:
- Assess the patient's satisfaction with their contraceptive method and whether they have any concerns about method use.
- Assess any changes in health status, including medications, that would change the appropriateness of POPs for safe and effective continued use on the basis of U.S. MEC (e.g., category 3 and 4 conditions and characteristics).[1]
- Consider assessing weight changes and discussing concerns about any changes in weight and whether changes might be related to use of the contraceptive method.
- Assess the patient's satisfaction with their contraceptive method and whether they have any concerns about method use.
Comments and Evidence Summary
No evidence was found regarding whether a routine follow-up visit after initiating POPs improves correct or continued use.
Missed POPs
Norethindrone or Norgestrel POPs
For norethindrone or norgestrel POPs, a dose is considered missed if it has been >3 hours since it should have been taken. Recommendations are provided for missed norethindrone or norgestrel POPs (Figure 5).
Comments and Evidence Summary
Inconsistent or incorrect use of oral contraceptive pills is a major reason for oral contraceptive failure. Unlike COCs, which inhibit ovulation as the primary mechanism of action, norethindrone or norgestrel POPs inhibit ovulation in about half of cycles, although this rate varies widely by person.[333] Peak serum steroid levels are reached about 2 hours after administration, followed by rapid distribution and elimination, such that by 24 hours after administration, serum steroid levels are near baseline.[333] Therefore, taking norethindrone or norgestrel POPs at approximately the same time each day is important. An estimated 48 hours of norethindrone or norgestrel POP use was deemed necessary to achieve the contraceptive effects on cervical mucus.[333] For patients who frequently miss norethindrone or norgestrel POPs, explore patient goals, consider offering counseling on alternative contraceptive methods, and initiate another method if it is desired. No evidence was found regarding the effects of missed norethindrone or norgestrel POPs available in the United States on measures of contraceptive effectiveness including pregnancy, follicular development, hormone levels, or cervical mucus quality.
Figure 5. Recommended actions after late or missed progestin-only pills
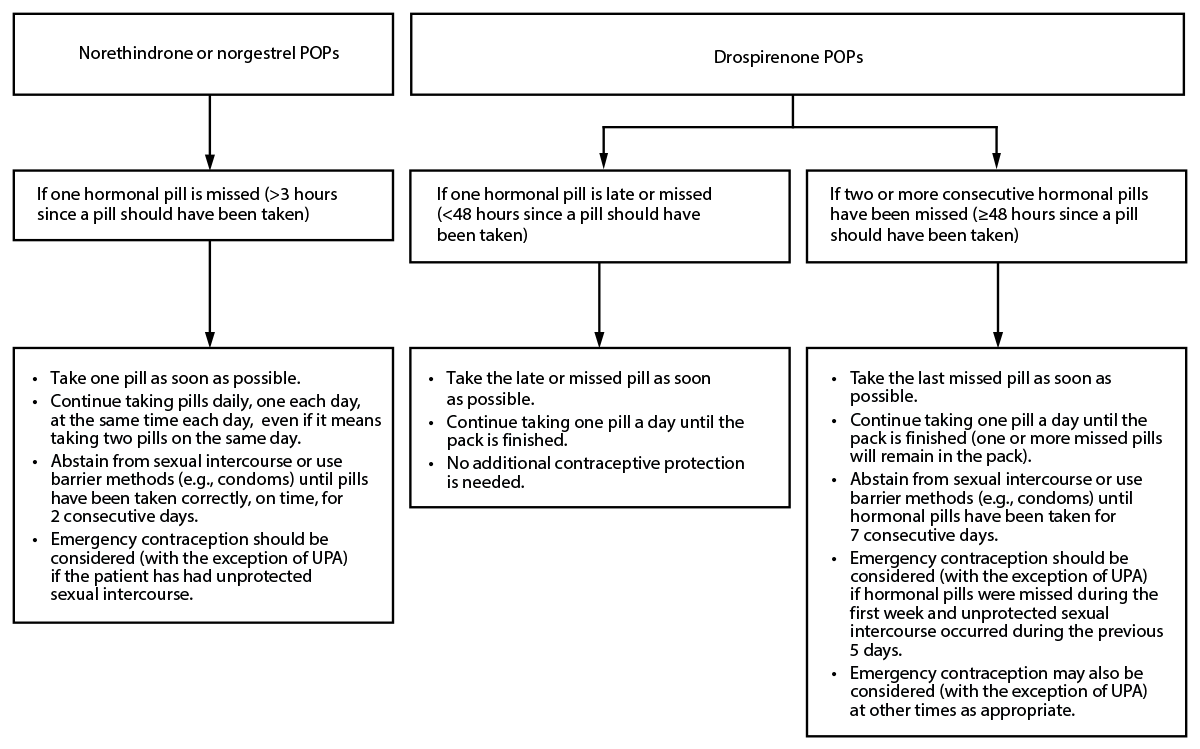
Abbreviations: POP = progestin-only pill; UPA = ulipristal acetate.
DRSP POPs
For the following recommendations, a dose is considered late when <24 hours have elapsed since the dose should have been taken. A dose is considered missed if ≥24 hours have elapsed since the dose should have been taken. For example, if a DRSP POP was supposed to have been taken on Monday at 9:00 a.m. and is taken at 11:00 a.m., the pill is late; however, by Tuesday morning at 11:00 a.m., Monday's 9:00 a.m. pill has been missed and Tuesday's 9:00 a.m. pill is late. For DRSP POPs, the recommendations only apply to late or missed hormonally active pills and not to placebo pills. Recommendations are provided for late or missed DRSP POPs (Figure 5).
Comments and Evidence Summary
Inconsistent or incorrect use of oral contraceptives is a major cause of oral contraceptive failure. Unlike norethindrone and norgestrel POPs, the primary mechanism of contraceptive effectiveness of DRSP POPs is ovulation inhibition. In a study of 27 patients receiving DRSP POPs in a regimen of 24 days of active pills/4 days of placebo pills, no subjects met normal ovulatory criteria over two treatment cycles.[334] Earliest time to ovulation resumption was day 9 after two 24/4 cycles were completed (day 13 after the last hormonally active pill was taken); mean time to ovulation after two 24/4 cycles were completed was 13.6±3.8 days.[334] In an RCT of 127 participants, participants purposefully missed pills (22–25 hour delay) on days 3, 6, 11, and 22 in either treatment cycle one or two of the 24/4 regimen.[335] Escape ovulation occurred in only one person over the two treatment cycles (ovulation incidence 0.8%; 95% CI 0%–4.4%).[335] DRSP has a half-life of approximately 30 hours with near-complete elimination by 10 days.[336] For patients who frequently miss DRSP POPs, explore patient goals, consider offering counseling on alternative contraceptive methods, and initiate another method if it is desired.
Vomiting or diarrhea (for any reason or duration) that occurs within 3 hours after taking a pill
Norethindrone or Norgestrel POPs
- Take another pill as soon as possible (if possible, despite discomfort).
- Continue taking pills daily, one each day, at the same time each day.
- Abstain from sexual intercourse or use barrier methods (e.g., condoms) until 2 days after vomiting or diarrhea has resolved.
- Emergency contraception should be considered (with the exception of UPA) if the patient has had unprotected sexual intercourse.
DRSP POPs
- Take another pill as soon as possible (if possible, despite discomfort).
- Continue taking pills daily, one each day, at the same time each day.
- If vomiting or diarrhea continues for >24 hours, then abstain from sexual intercourse or use barrier methods (e.g., condoms) for 7 days after vomiting or diarrhea has resolved.
- Emergency contraception should be considered (with the exception of UPA) if the patient has had unprotected sexual intercourse.
Comments and Evidence Summary
Theoretically, the contraceptive effectiveness of all POPs might be decreased because of vomiting or diarrhea. Because of the lack of evidence to address this question, these recommendations are based on the recommendations for missed POPs. No evidence was found regarding the effects of vomiting or diarrhea on measures of contraceptive effectiveness, including pregnancy, follicular development, hormone levels, or cervical mucus quality.
