At a glance
- National ALS Biorepository collects biosamples from consented participants of the Registry
- Deidentified biosamples are available only for approved research studies

What is the National ALS Biorepository?
The National ALS Biorepository is part of the National ALS Registry. This Biorepository will include samples from persons living with ALS who are enrolled in the National ALS Registry. ATSDR developed a plan for creating this Biorepository with help from external experts. This plan describes the best ways to collect, store, and share biological samples. Persons living with ALS must give their consent to take part in the National ALS Biorepository.
This Biorepository may help scientists better understand the cause(s) of ALS. Researchers may be able to study the genetic variation in those with ALS. The National ALS Registry collects epidemiological data from persons living with ALS. Connecting biological samples with these data will make the National ALS Registry more complete and useful.
National ALS Biorepository Samples
Persons living with ALS
Persons living with ALS must be enrolled in the National ALS Registry to take part in the Biorepository. After an individual has joined the Registry, he/she will be able to ask for more information about the Biorepository and provide his/her contact information. To learn more about this process click here.
Specimens that may be collected for each component of the Biorepository are below.
- The premortem (in-home) part involves the collection of blood, urine, hair and fingernail clipping specimens collected in their homes.
- The postmortem part involves the donation of brain; spinal cord; cerebral spinal fluid; and pieces of muscle, skin, and bone from persons living with ALS after they have died.
ALS Researchers
Researchers can request samples for their research. Samples are available to researchers around the globe regardless of institutional affiliation. The samples come from persons living with ALS who have enrolled in the National ALS Registry and, when available, can be linked to epidemiological data (e.g., smoking history, residential history, occupational history, and history of military service) collected by the Registry.
General flowchart of research application process
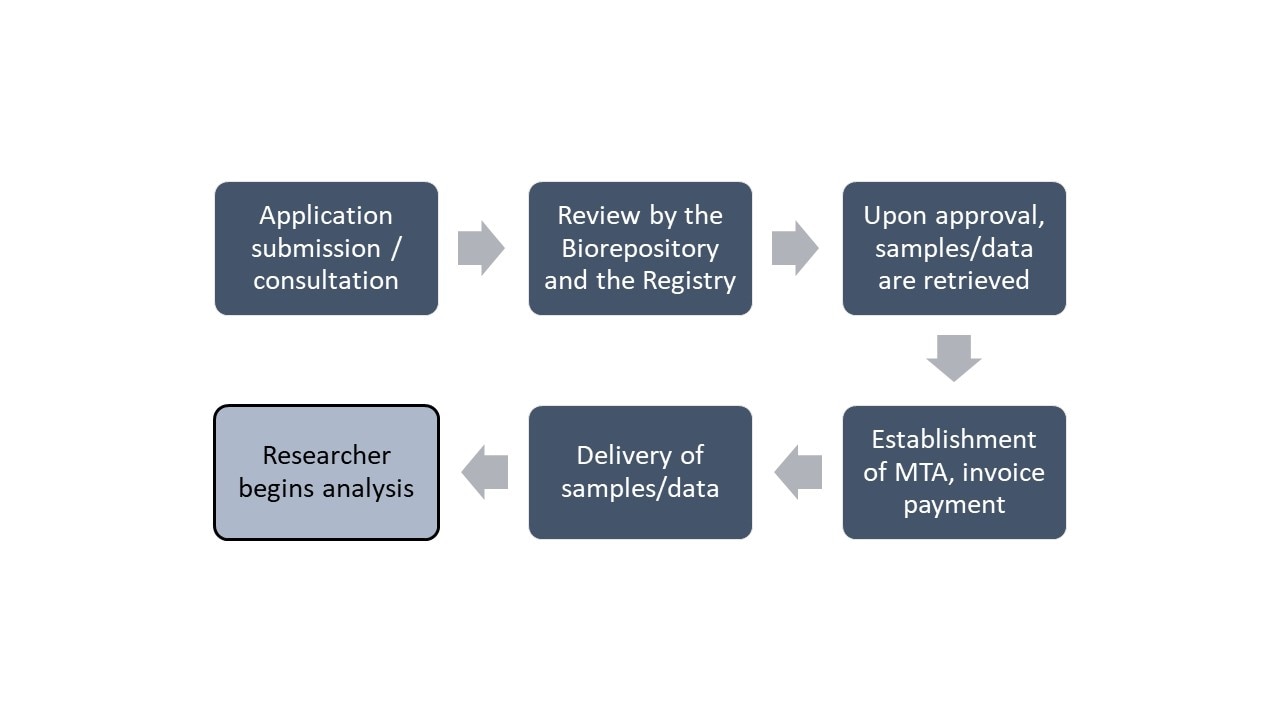
ATSDR will not consider any research that does not have IRB approval and an FDA IND or IDE number for an investigational test article, if applicable. Any request for samples or data requires application completion. Once submitted, ATSDR will review the application for completeness, sample availability, and relevance to ALS research. It will then be evaluated by an internal/external scientific panel. In addition to the standard review, ATSDR will also review the application for sample availability and appropriateness of sample use. To learn more about the sample inventory and application process, click here.
Due to the complex variations in specimen and epidemiologic data availability, we highly encourage you to contact one of our registry specialists to discuss your data and specimen needs before proposing any studies using the Registry. If you are submitting a grant proposal, please contact the Extramural Research Program Office (ERPO) for guidance. If you are submitting a grant proposal for another federal agency, please contact the National ALS Biorepository at the number below.
Contacts
If you have questions about the National ALS Biorepository please call 1-855-874-6912 or email questions to alsbiorepository@secure.mcking.com (Monday through Friday from 8:30am to 5pm ET).
If you have questions about the National ALS Registry please call 1-877-442-9719 (Monday through Friday from 8am to 5pm ET).
