At a glance
The Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination to all adults aged ≥65 years (1). The purpose of this study was to assess potential effect of the COVID-19 pandemic on pneumococcal vaccination coverage (≥1 dose of any type of pneumococcal vaccine) among adults aged 65 through 70 years.
Summary
The Advisory Committee on Immunization Practices (ACIP) recommends pneumococcal vaccination to all adults aged ≥65 years1. The purpose of this study was to assess potential effect of the COVID-19 pandemic on pneumococcal vaccination coverage (≥1 dose of any type of pneumococcal vaccine) among adults aged 65 through 70 years.
To assess pneumococcal vaccination coverage among adults aged 65–70 years, the Centers for Disease Control and Prevention (CDC) analyzed data from the 2015–2021 National Health Interview Survey (NHIS)23 and Behavioral Risk Factor Surveillance System (BRFSS)45. Each year, respondents were asked: “Have you ever had a pneumonia shot?”. Pneumococcal vaccination coverage by age in years (65 through 70 years) was assessed, stratified by year of birth (1948–1956). We mainly emphasized and compared estimates in 2018 with estimates in 2020-21 given the change in the recommendations in 20196.
Pneumococcal vaccination coverage among adults who reached 65–70 years of age during the pandemic (2020–2021 combined) based on BRFSS was 61.0%, 3.3 percentage points lower compared with coverage among those who reached 65–70 years of age before the pandemic (in 2018) and coverage was usually lower when compared with the estimates assessed in 2017, 2016, and 2015; coverage during the pandemic in 2020 and 2021 based on BRFSS was 3.6 and 2.9 percentage points lower, respectively, compared with coverage before the pandemic (in 2018). However, there was generally no significant difference in coverage in 2020–2021 (combined) compared with the estimate in 2018 based on data from the NHIS.
Pneumococcal vaccination coverage among adults aged ≥65 years is suboptimal. Both reductions in persons accessing vaccination services and changes in vaccination recommendations may have contributed to lower coverage during the COVID-19 pandemic. Providers assessing the vaccination status of all patients at each medical visit to avoid missed opportunities for vaccination is essential to ensure timely vaccine catch-up. More efforts are needed to educate providers about the new recommendations, and to work with health systems and provider practices to operationalize these new recommendations.
Methods
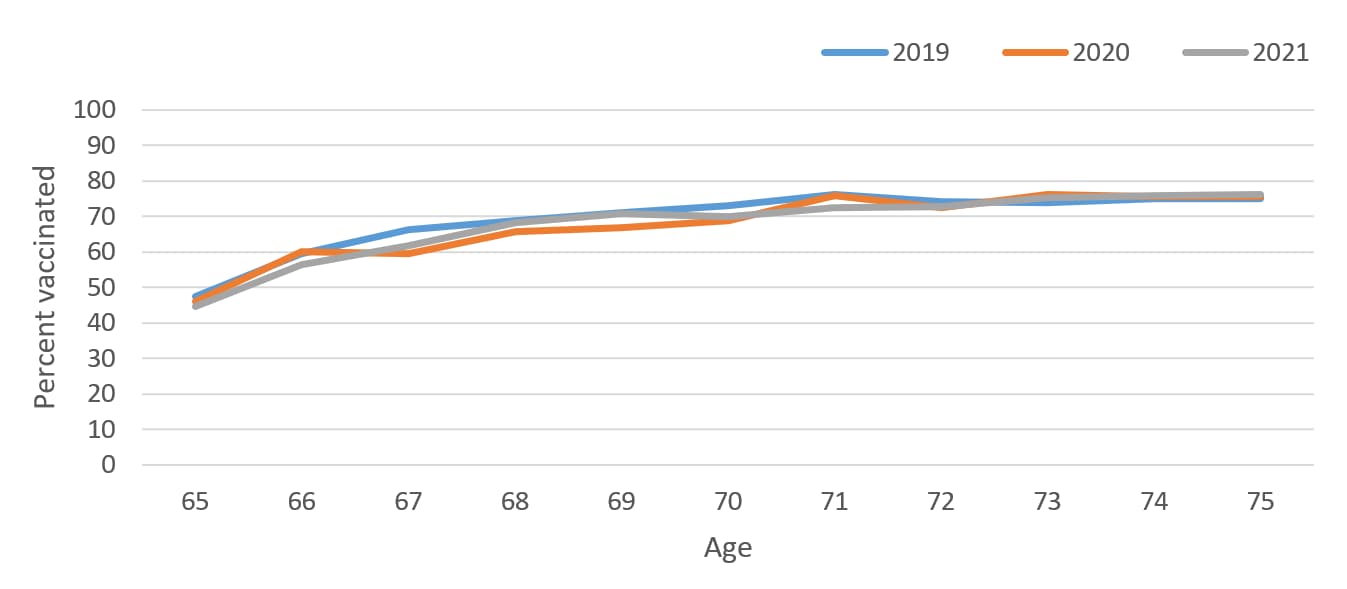
To determine the appropriate age group for this assessment, we looked at pneumococcal vaccination by single year of age from 65 through 75 years for persons born in 1944–1956 using data from years 2019–2021 to assess the cumulative vaccine uptake by age. Based on this assessment, we chose to focus the assessment of potential pandemic effects on ages 65–70 years, during which the majority of pneumococcal vaccinations were given, after which coverage then plateaued (Figure 1, Figure 2).
Figure 1. Pneumococcal vaccination coverage by single year of age from 65-75 years for persons born 1944–1956, National Health Interview Survey, 2019–2021
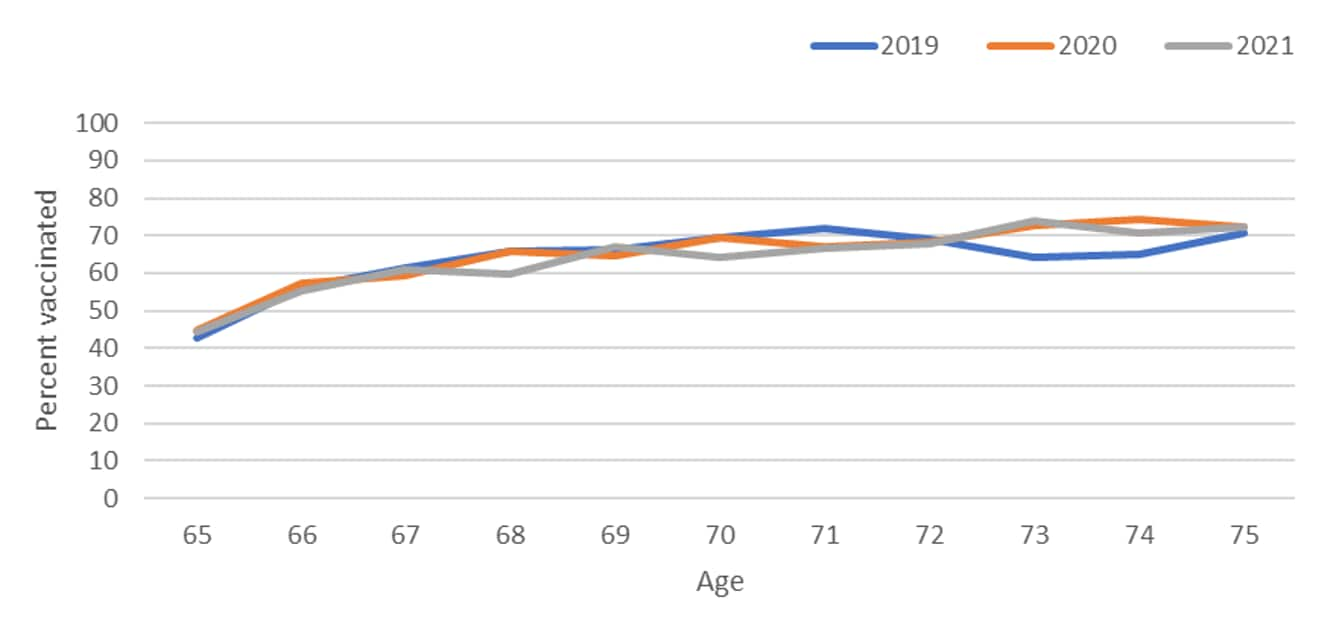
Figure 2. Pneumococcal vaccination coverage by single year of age from 65–75 years for persons born 1944–1956, Behavioral Risk Factor Surveillance Survey, 2019–2021
To assess the potential impact of the COVID-19 pandemic, pneumococcal vaccination coverage among adults aged 65–70 years was analyzed using data from the 2015–2021 NHIS and BRFSS. To assess the difference in vaccine coverage before and after COVID-19 pandemic, we used vaccine coverage estimates in 2018 and 2020–2021 as the primary comparison given the changes in the pneumococcal vaccine recommendations in 20196. Prior to 2019, all adults aged ≥65 years were recommended to receive both the 13-valent pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal polysaccharide vaccine (PPSV23); in 2019, the recommendation was updated and routine PCV13 was no longer recommended for adults aged ≥65 years without an immunocompromising condition, cochlear implant, or cerebrospinal fluid leak. The NHIS is an annual household survey conducted by the U.S. Census Bureau for CDC’s National Center for Health Statistics23. NHIS provides estimates on health indicators, healthcare utilization and access, and health-related behaviors for the U.S. resident civilian noninstitutionalized population. The NHIS sample was selected using a complex sampling design involving stratification, clustering, and multistage sampling with a nonzero probability of selection for each person. Face-to-face interviews were conducted in a probability sample of households. However, NHIS data from 2020 at the start of the COVID-19 pandemic and from January through April 2021 were obtained by telephone rather than in-person interviews. In the sample adult component, one adult per sampled family was randomly selected and asked to complete the sample adult questionnaire. The sample adult core survey included questions on pneumococcal vaccination. Respondents were asked: “Have you ever had a pneumonia shot?” Respondents who answered “yes” were considered vaccinated. The final response rates in 2015–2021 for the core survey sample of adults ranged from 48.9% to 61.1%23
The BRFSS is a continuous, population-based telephone survey of noninstitutionalized adults aged ≥18 years coordinated by state health departments in collaboration with CDC. The BRFSS collects information on health conditions and risk behaviors from randomly selected adults. Respondents were asked “Have you ever had a pneumonia shot, also known as a pneumococcal vaccine?” Respondents who answered “yes” were considered vaccinated. Response rates for BRFSS are calculated using standards set by the American Association for Public Opinion Research. The landline–cellular phone combined median response rates in 2015–2021 BRFSS ranged from 44.0% to 49.9%45
Pneumococcal vaccination coverage by age in years (65 through 70 years) was assessed, stratified by year of birth (1948–1956). To assess potential COVID-19 pandemic effects on pneumococcal vaccination by age, differences in vaccination coverage among those reaching the target age in 2018 and those reaching the age in 2020 and 2021 were assessed. For example, vaccination coverage by age 65 years was compared for adults born in 1955 and 1956 (reached age 65 years in 2020 and 2021, during the pandemic) with those born in 1953 (reached age 65 years in 2018, pre-pandemic); vaccination coverage by age 66 years was compared for adults born in 1954 and 1955 (reached age 66 years in 2020 and 2021, during the pandemic) with those born in 1952 (reached age 66 years in 2018, pre-pandemic); and vaccination coverage by ages 67, 68, 69, and 70 years followed the same pattern. Reference birth years for vaccination coverage difference estimates by ages 67, 68, 69, and 70 years were the 1951, 1950, 1949, and 1948 birth years, respectively. Adults in these reference birth years reached the given age in 2018 (e.g., for coverage by age 67 years, adults born in 1951 reached 67 in 2018, which was pre-pandemic). We also compared the differences in vaccine coverage using 2015, 2016, 2017, and 2019 as pre-pandemic reference years. SUDAAN statistical software (version 11; RTI International), a statistical tool for complex sample surveys (Research Triangle Institute, Research Triangle Park, NC) was used to calculate point estimates and 95% confidence intervals (CI). All analyses were weighted to reflect the age, sex, and race/ethnicity of the U.S. noninstitutionalized civilian population.
Results
Based on data from the NHIS, pneumococcal vaccination coverage among adults who reached 65–70 years of age during the pandemic (2020–2021 combined) was 59.3%, similar to coverage before the pandemic (in 2018, 61.2%) and coverage was generally not significantly different when compared with the estimates assessed in 2017, 2016, and 2015 (Table 1, Table 2). There were no significant differences in pneumococcal vaccination coverage among adults who reached any individual year of age from 65–70 during the pandemic (2020, 2021, or 2020–2021 combined) compared with coverage before the pandemic (in 2018) (Table 1, Table 2).
Table 1. Report of any prior pneumococcal vaccination (≥1 dose) by age and birth year 1948-1956, National Health Interview Survey 2015-2021*
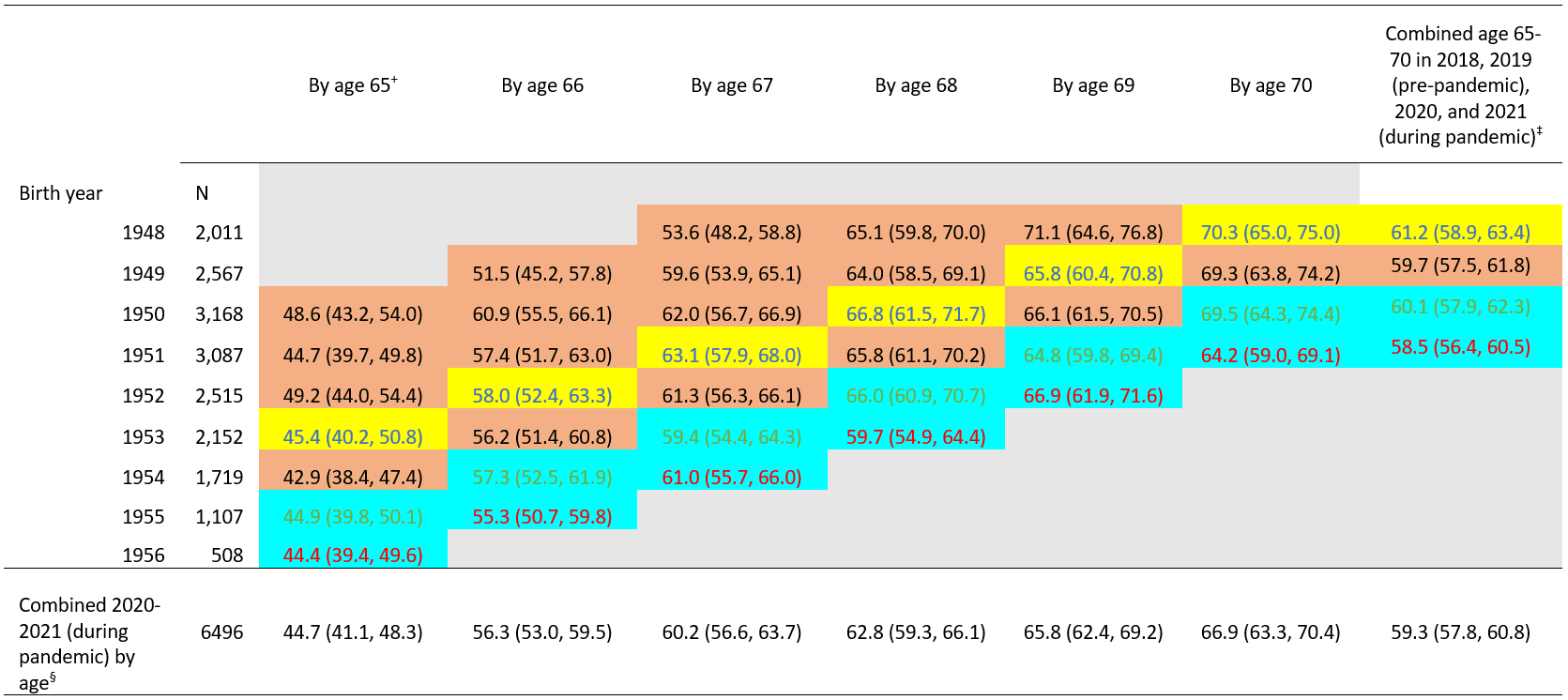
*Table cells are shaded according to whether persons with the indicated birth year (rows) reached the age indicated (columns) in 2015–2017 and 2019 or 2018 (pre-pandemic, shaded in orange or yellow) or in 2020 or 2021 (during pandemic, shaded in blue). Cells shaded in yellow were used as the pre-pandemic referent cell (those reaching the age in 2018) for comparisons with pandemic cells, for each age indicated (column).
+ Age based on year of birth and survey year. For example, data for persons born in 1950 who reached age 65 were obtained from the 2015 NHIS, and some could have still been age 64 when interviews were conducted.
‡ Combined age 65–70 in 2018 or 2019 (pre-pandemic, shaded in yellow or orange; n=2,718 for 2018 and n=3,365 for 2019) and 2020 or 2021 (during pandemic, shaded in blue; n=3,509 for 2020 and n=2,987 for 2021).
§ Combined 2020–2021 (during pandemic) by age, persons with the indicated birth year (rows) reached the age indicated (columns) in 2020–2021 (combined two blue cell shadings) (e.g., for coverage by age 65 years, people born in 1955 or 1956 reached 65 in 2020 or 2021, which was during pandemic).
Table 2. Difference in vaccine coverage of pneumococcal vaccination (≥1 dose) by age and birth year 1948–1956, National Health Interview Survey 2015–2021*
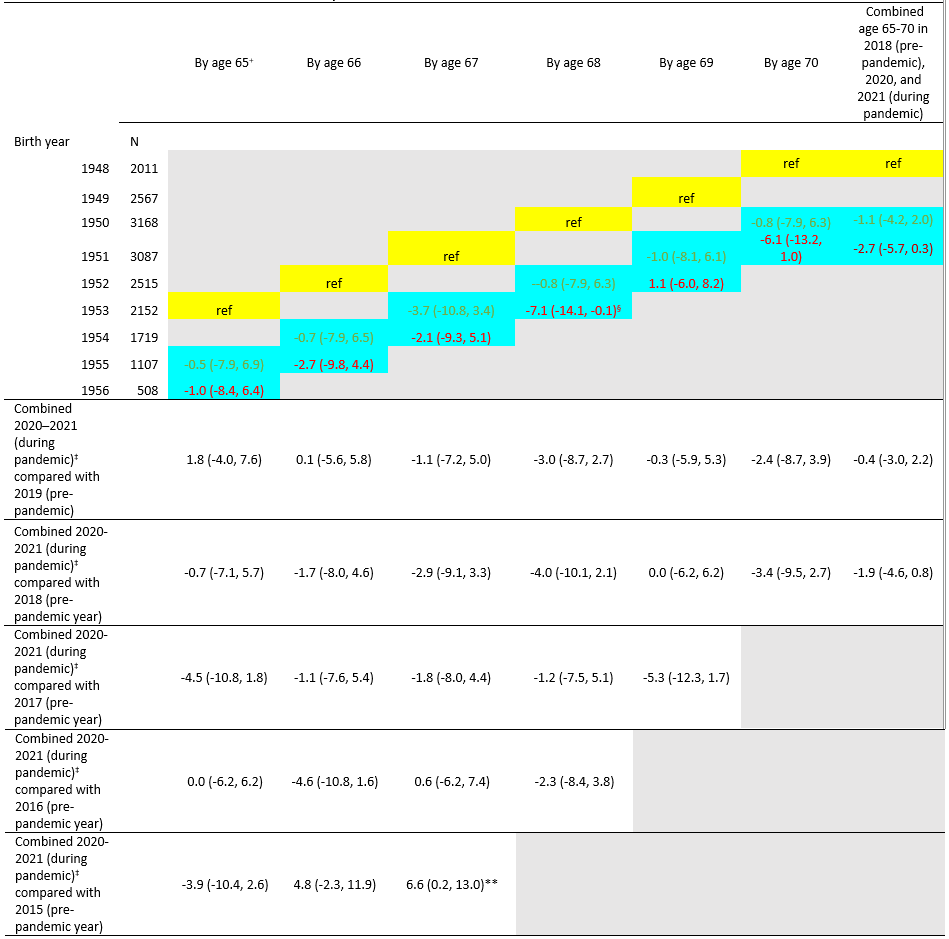
* People in these reference birth years reached the given age in 2018 (pre-pandemic year) (e.g., for coverage by age 66 years, people born in 1952 reached 66 in 2018, which was before the pandemic). The pre-pandemic year (reference: yellow cell shading) is compared to pandemic years (2020 and 2021, blue cell shading; or 2020–2021, combined two blue cell shadings).
† Age based on year of birth and survey year. For example, data for persons born in 1950 who reached age 65 were obtained from the 2015 NHIS, and some could have still been age 64 when interviews were conducted.
‡ Combined 2020–2021 (during pandemic) by age, persons with the indicated birth year (rows) reached the age indicated (columns) in 2020–2021 (combined two blue cell shadings) (e.g., for coverage by age 65 years, people born in 1955 or 1956 reached 65 in 2020 or 2021, which was during pandemic).
§ p<0.05 by t-test for comparisons within each age with the reference level (2018 estimate for each age) as indicated.
** p<0.05 by t-test for comparisons within each age with the reference level (2019, 2017, 2016, 2015 estimates for each age) as indicated.
Based on data from the BRFSS, pneumococcal vaccination coverage among adults who reached 65–70 years of age during the pandemic (2020–2021 combined) was 61.0%, 3.3 percentage points lower compared with coverage before the pandemic (in 2018) and coverage was lower for most age in years when compared with the estimates assessed in 2017, 2016, and 2015; coverage during the pandemic in 2020 and 2021 was 3.6 and 2.9 percentage points lower, respectively, compared with coverage before the pandemic (in 2018) (Table 3, Table 4). Pneumococcal vaccination coverage among adults who reached 65, 66, 67, 68, 69, or 70 years of age during the pandemic (2020–2021 combined) was 0.1-5.7 percentage points lower compared with coverage before the pandemic (in 2018). The largest differences were seen among adults who reached age 67 years during the pandemic. Pneumococcal vaccination coverage was 7.0 (95% CI: -10.5, -3.5) and 4.5 (95% CI: -7.8, -1.2) percentage points lower among adults who reached 67 years of age in 2020 and 2021, respectively than those who reached 67 years of age before the pandemic (in 2018) (Table 3, Table 4).
Table 3. Report of any prior pneumococcal vaccination (≥1 dose) by age and birth year 1948–1956, Behavioral Risk Factor Surveillance System 2015–2021*
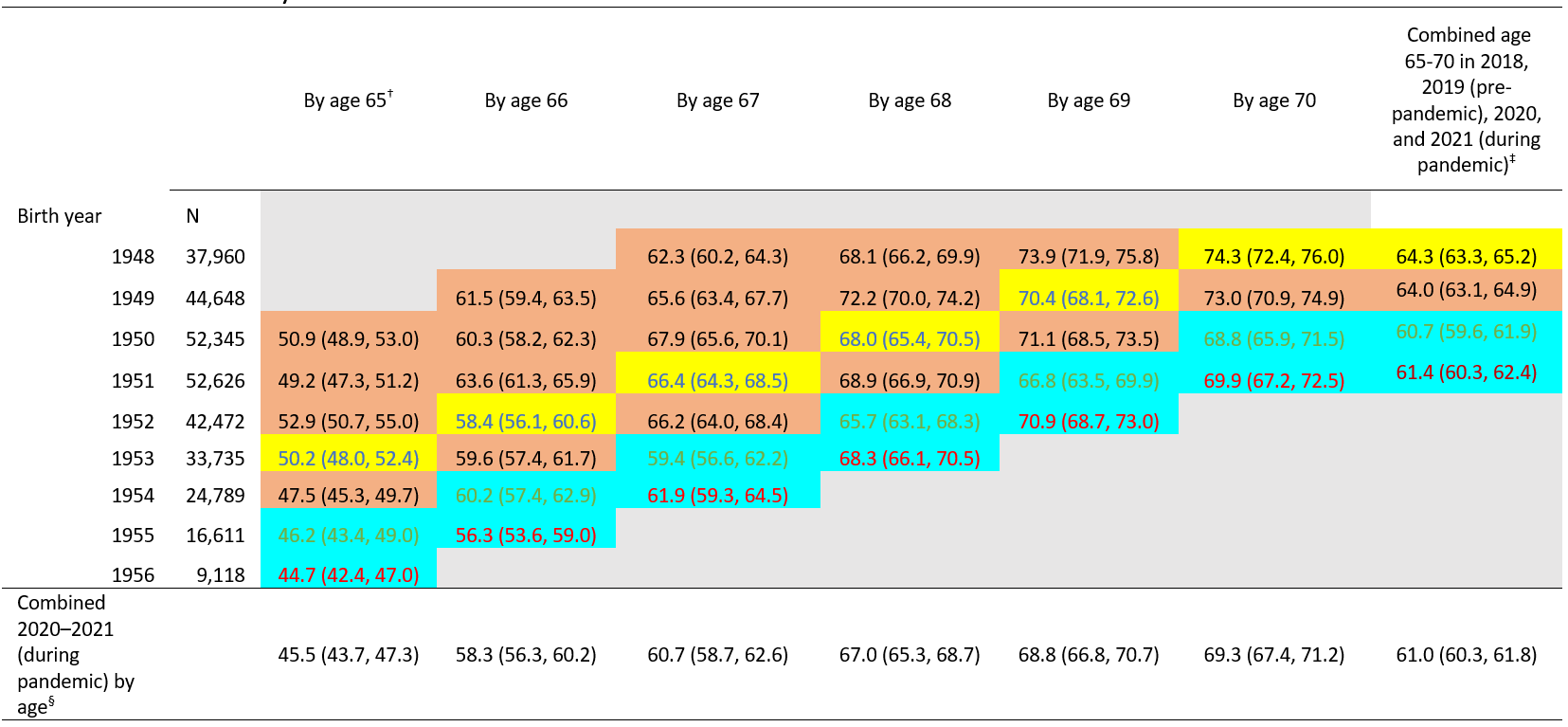
*Table cells are shaded according to whether persons with the indicated birth year (rows) reached the age indicated (columns) in 2015–2017 and 2019 or 2018 (pre-pandemic, shaded in orange or yellow) or in 2020 or 2021 (during pandemic, shaded in blue). Cells shaded in yellow were used as the pre-pandemic referent cell for comparisons with pandemic cells, for each age indicated (column).
† Age based on year of birth and survey year. For example, data for persons born in 1950 who reached age 65 were obtained from the 2015 BRFSS, and some could have still been age 64 when interviews were conducted.
‡ Combined age 65–70 in 2018 or 2019 (pre-pandemic, shaded in yellow or orange; n=54,257 for 2018 and n=49,526 for 2019) and 2020 or 2021 (during pandemic, shaded in blue; n=46,123 for 2020 and n=50,552 for 2021).
§ Combined 2020–2021 (during pandemic) by age, persons with the indicated birth year (rows) reached the age indicated (columns) in 2020–2021 (combined two blue cell shadings) (e.g., for coverage by age 65 years, people born in 1955 or 1956 reached 65 in 2020 or 2021, which was during pandemic).
Table 4. Difference in uptake of pneumococcal vaccination (≥1 dose) by age and birth year 1948–1956, Behavioral Risk Factor Surveillance System 2015–2021*

*People in these reference birth years reached the given age in 2018 (pre-pandemic year) (e.g., for coverage by age 66 years, people born in 1952 reached 66 in 2018, which was before the pandemic). The pre-pandemic year (reference: yellow cell shading) is compared to pandemic years (2020 and 2021, blue cell shading; or 2020–2021, combined two blue cell shadings).
† Age based on year of birth and survey year. For example, data for persons born in 1950 who reached age 65 were obtained from the 2015 BRFSS, and some could have still been age 64 when interviews were conducted.
‡ p<0.05 by t-test for comparisons within each age with the reference level (2018 estimate for each age) as indicated.
§ Combined 2020–2021 (during pandemic) by age, persons with the indicated birth year (rows) reached the age indicated (columns) in 2020–2021 (combined two blue cell shadings) (e.g., for coverage by age 65 years, people born in 1955 or 1956 reached 65 in 2020 or 2021, which was during pandemic).
** p<0.05 by t-test for comparisons within each age with the reference level (2019, 2017, 2016, 2015 estimates for each age) as indicated.
Discussion
While overall pneumococcal vaccination coverage among adults aged ≥65 years is suboptimal, disruptions to primary care reported during the pandemic may have led to further reductions in coverage 7. Previous studies have identified declines in routine adolescent vaccination coverage during the pandemic8 and in routine pediatric vaccine ordering and doses administered early in the pandemic910. Many older adults may have missed routine medical care and recommended vaccinations during the COVID-19 pandemic in 2020 and 2021. Unlike children, among whom coverage for most routinely recommended vaccines was generally high prior to the COVID-19 pandemic11, coverage with routinely recommended vaccines among adults has historically been low12. The COVID-19 pandemic may have contributed to decreases in already suboptimal coverage. Data from the BRFSS indicated that there might have been a modest decrease in pneumococcal vaccination among adults turning 65–70 years of age during the pandemic, with coverage 3.3 (range: 0.1-5.7) percentage points lower compared with 2018. Even though routine medical care visits generally resumed by 2021, the decrease in pneumococcal vaccination persisted in 2021. Coverage decrease might be due to COVID-19-related reductions in persons accessing vaccination services and vaccination recommendations changed around the same time.
Since 1980s, ACIP has recommended the 23-valent pneumococcal polysaccharide vaccine (PPSV23) to all adults aged ≥65 years13. In 2014, ACIP recommended routine use of the 13-valent pneumococcal conjugate vaccine (PCV13) in series with PPSV23 for all adults aged ≥65 years14. In 2019, ACIP removed the recommendation for routine PCV13 use among adults aged ≥65 years without an immunocompromising condition, cochlear implant, or cerebrospinal fluid leak but recommended all adults aged ≥65 years should continue to receive 1 dose of PPSV236. In 2021, ACIP recommended 15-valent pneumococcal conjugate vaccine (PCV15) followed by PPSV23 or 20-valent pneumococcal conjugate vaccine (PCV20) alone for adults aged ≥65 years15. Routine vaccination is an essential preventive care service for children, adolescents, and adults that should not be delayed because of the COVID-19 pandemic. Guidance on resuming safe vaccination of adults is available1617. Many of the long-established public health actions to increase vaccination coverage among adults can be used for routine vaccinations. Sending reminders to patients about needed vaccines, assessing the vaccination status of all patients at each visit to avoid missed opportunities for vaccination, and recommending and offering or referring patients for needed vaccinations can help improve timely vaccine catch-up1819. Additionally, to improve pneumococcal vaccination coverage, the PneumoRecs VaxAdvisor mobile app might be used by providers and help vaccination providers quickly and easily determine which pneumococcal vaccines a patient needs and when20.
Limitations
The findings in this report are subject to several limitations. First, vaccination coverage was self-reported and therefore might be subject to recall bias or social desirability bias. However, adult self-reported vaccination status for pneumococcal vaccination has been shown to be sensitive and specific21. Second, the BRFSS is a telephone survey, which excludes people without telephones. Third, the 2015–2021 response rates ranged from 48.9% to 61.1% for the NHIS, and 44.0% to 49.9% for the BRFSS, and nonresponse bias may remain if survey weighting does not fully correct for this. Fourth, both the NHIS and BRFSS excluded persons in the military and those residing in institutions, which might result in underestimation or overestimation of vaccination coverage levels. However, none of the aforementioned limitations differed before and during the pandemic. Fifth, based on the way that the question is worded (Have you ever had a pneumonia shot?), people could have had vaccination before age 65 years as well and we could not assess impact of the pandemic on being up to date with the recommended number and type of pneumococcal vaccinations even though many adults aged ≥65 years with a medical condition were recommended to receive more than one pneumococcal vaccine based on recommendations during 2019-2021. Finally, we used cross-sectional data to approximate pneumococcal vaccination coverage by age among birth cohorts, which did not take into account mortality, emigration, or immigration.
- Adult immunization schedule by age. Available at: https://www.cdc.gov/vaccines/hcp/imz-schedules/adult-age.html. Accessed March 17, 2023.
- National Center for Health Statistics. National Health Interview Survey. Public-use data file and documentation. https://www.cdc.gov/nchs/nhis/documentation/index.html. Accessed March 17, 2023.
- National Health Interview Survey. Available at: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2021/srvydesc-508.pdf. Accessed June 16, 2023.
- BRFSS: overview– BRFSS 2021. Available at: https://www.cdc.gov/brfss/annual_data/2021/pdf/Overview_2021-508.pdf. Accessed June 16, 2023.
- BRFSS annual survey data. Available at: https://www.cdc.gov/brfss/annual_data/annual_data.htm. Accessed March 17, 2023.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2019;68:1068–1075.
- Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19–related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep 2020;69(36):1250–1257.
- Estimated routine vaccination coverage (≥1 HPV vaccine dose, ≥1 Tdap, ≥1 MenACWY) among adolescents by age and birth year, National Immunization Survey-Teen, 2015-2021. Available at: https://www.cdc.gov/teenvaxview/publications/nis-teen-coverage-estimates-2015-2021.html. Accessed March 23, 2023.
- Santoli JM, Lindley MC, DeSilva MB, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69(19):591–593.
- Hong K, Zhou F, Tsai Y, et al. Decline in Receipt of Vaccines by Medicare Beneficiaries During the COVID-19 Pandemic — United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:245–249.
- ChildVaxView. Available at: https://www.cdc.gov/childvaxview/index.html. Accessed April 25, 2023.
- Lu PJ, Hung MC, Srivastav A, et al. Surveillance of Vaccination Coverage Among Adult Populations -United States, 2018. MMWR Surveill Summ. 2021 May 14;70(3):1-26.
- Recommendations of the Immunization Practices Advisory Committee (ACIP) update: pneumococcal polysaccharide vaccine usage — United States. MMWR Recomm Rep 1984 / 33(20);273-276.
- Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014;63:822–5.
- Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-Valent Pneumococcal Conjugate Vaccine and 20-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:109–117.
- Routine immunizations on schedule for everyone (RISE). Available at: https://www.cdc.gov/vaccines/php/rise/. Accessed April 14, 2023.
- Vaccination guidance during a pandemic. Atlanta, GA: US Department of Health and Human Services, CDC, 2020. Available at: https://www.cdc.gov/vaccines/pandemic-guidance/index.html. Accessed March 23, 2023.
- National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory committee: standards for adult immunization practice. Public Health Rep. 2014;129(2):115-123.
- Guide to Community Preventive Services. Available at: https://www.thecommunityguide.org. Accessed May 22, 2023.
- PneumoRecs VaxAdvisor Mobile App for vaccine providers. Available at: https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/app.html. Accessed June 1, 2023.
- Rolnick SJ, Parker ED, Nordin JD, et al. Self-report compared to electronic medical record across eight adult vaccines: do results vary by demographic factors? Vaccine 2013;31(37):3928–3935.
