
Risk Prediction Score for Chronic Kidney Disease in Healthy Adults and Adults With Type 2 Diabetes: Systematic Review
SYSTEMATIC REVIEW — Volume 20 — April 20, 2023
Alejandra González-Rocha, MS1; Victor A. Colli, MD1,2; Edgar Denova-Gutiérrez, PhD1 (View author affiliations)
Suggested citation for this article: González-Rocha A, Colli VA, Denova-Gutiérrez E. Risk Prediction Score for Chronic Kidney Disease in Healthy Adults and Adults With Type 2 Diabetes: Systematic Review. Prev Chronic Dis 2023;20:220380. DOI: http://dx.doi.org/10.5888/pcd20.220380.
PEER REVIEWED
What is already known on this topic?
Previous reviews found 30 models to identify chronic kidney disease (CKD) in healthy populations, some with good discriminatory performance.
What is added by this report?
Our study identified 36 models for risk of CKD in healthy populations and 12 in populations with type 2 diabetes. We found 13 models with good discriminatory performance for healthy populations and 4 for populations with type 2 diabetes.
What are the implications for public health practice?
These models could be tools for preventing CKD. Some of them could be the base to develop a tool for use in primary care settings.
Abstract
Introduction
Chronic kidney disease (CKD) is an important public health problem. In 2017, the global prevalence was estimated at 9.1%. Appropriate tools to predict the risk of developing CKD are necessary to prevent its progression. Type 2 diabetes is a leading cause of CKD; screening the population living with the disease is a cost-effective solution to prevent CKD. The aim of our study was to identify the existing prediction scores and their diagnostic accuracy for detecting CKD in apparently healthy populations and populations with type 2 diabetes.
Methods
We conducted an electronic search in databases, including Medline/PubMed, Embase, Health Evidence, and others. For the inclusion criteria we considered studies with a risk predictive score in healthy populations and populations with type 2 diabetes. We extracted information about the models, variables, and diagnostic accuracy, such as area under the receiver operating characteristic curve (AUC), C statistic, or sensitivity and specificity.
Results
We screened 2,359 records and included 13 studies for healthy population, 7 studies for patients with type 2 diabetes, and 1 for both populations. We identified 12 models for patients with type 2 diabetes; the range of C statistic was from 0.56 to 0.81, and the range of AUC was from 0.71 to 0.83. For healthy populations, we identified 36 models with the range of C statistics from 0.65 to 0.91, and the range of AUC from 0.63 to 0.91.
Conclusion
This review identified models with good discriminatory performance and methodologic quality, but they need more validation in populations other than those studied. This review did not identify risk models with variables comparable between them to enable conducting a meta-analysis.
Introduction
Chronic kidney disease (CKD) has been defined as abnormalities of kidney structure or function present for more than 3 months (1). CKD is a public health problem (2–4). According to data from the Global Burden of Disease (GBD) study, in 2017 (4) the prevalence of CKD was estimated at 9.1% globally. Of total mortality, 4.6% of deaths were attributable to CKD and cardiovascular disease (CVD), which was attributable to impaired kidney function.
Type 2 diabetes became the second leading cause of CKD and CKD-related deaths in 2019 (3). Impaired fasting plasma glucose, high blood pressure, high body mass index, a diet high in sodium, and lead were risk factors for CKD quantified in GBD. Approximately 31% of CKD disability-adjusted life years were attributable to diabetes (4).
After automatic reporting of the glomerular filtration rate (eGFR) began, referrals to nephrology specialists by primary care services increased. However, the proportion of appropriate referrals did not change, indicating a need to develop appropriate screenings for CKD (5). Persons living with hypertension, diabetes, or cardiovascular diseases should be screened for CKD; identifying and treating CKD would reduce the burden of kidney disease (6). CKD can be detected early through inexpensive interventions (4).
Echouffo-Tcheugui and Kengne presented a systematic review with 30 models predicting the occurrence of CKD and concluded that some models had acceptable discriminatory performance (7). CKD screening in groups at high risk is likely to be cost-effective. Predictive models that incorporate clinical information systems would facilitate improved treatment allocations and health care management (6,8).
The aim of our study was to identify the existing prediction risk scores and their diagnostic accuracy for detecting CKD in apparently healthy adults and adults living with type 2 diabetes.
Methods
We followed the methodology proposed by the Cochrane handbook for systematic reviews of Diagnostic Test Accuracy (DTA). The protocol was published at PROSPERO (https://www.crd.york.ac.uk/prospero/), registration number CRD42021252888.
A search strategy was designed for the following databases: Cochrane Library, Medline/PubMed, Embase, Latin American and Caribbean Health Sciences Literature (LILACS), Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycInfo, Trip Database, Epistemonikos, and Health Evidence. We used the medical subject heading (MeSH) term “renal insufficiency, chronic” and the terms “risk models” and “predictive models,” which were validated in a pilot search. The detailed search strategy is in the Appendix. The databases used mostly had artificial intelligence that helped to mix these terms with similar terms. All the records were screened by title and abstract, then assessed by full text. We finally selected the ones that met all the selection criteria. The screening process included the reference list of the studies included in this review, other similar reviews, and a manual search of other studies identified for the authors in previous searches.
Study selection
The inclusion criteria included cohort and cross-sectional studies without language restrictions. The search was intentionally limited from May 2011 to November 2021 to update the information provided in previous reviews.
Studies that included healthy adults and adults living with type 2 diabetes were incorporated. The exclusion criteria were studies where the database used was from hospitalized patients with an initial diagnosis of CKD, and patients living with type 1 diabetes.
Included articles had to report models as a risk assessment tool that predicted CKD in healthy adults or adults living with type 2 diabetes. In this review we excluded predictive models of mortality, progression of CKD, and machine learning technology.
Major outcomes that we sought were area under the receiver operating characteristic curve (AUC) or C statistic to predict the presence or occurrence of CKD in healthy adults and adults with type 2 diabetes. Secondary outcomes that we looked for were sensitivity and specificity to predict the presence or occurrence of CKD in healthy adults and adults with type 2 diabetes. Studies that we included compare their models with reference standards eGFR, albuminuria, or proteinuria.
Two authors of this review (A.G.-R. and V.C.) independently screened titles and abstracts to identify relevant articles. In the first step of this process, reviews were removed, then full texts of the remaining articles were systematically examined for inclusion or exclusion. In the event of disagreement, the participation of the third author (E.D.-G.) was necessary to decide whether to include the article. The selection stages are shown in the flowchart based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1).

![]()
Figure 1.
Selection of studies process for analysis of chronic kidney disease (CKD) in healthy adults and adults living with type 2 diabetes. Abbreviations: CINAHL, Cumulative Index to Nursing and Allied Health Literature; CVD, cardiovascular disease; eGFR, glomerular filtration rate; ESRD, end-stage renal disease; LILACS, Latin American and Caribbean Health Sciences Literature. [A text description of this figure is also available.]
The information extracted was the design of the studies, type of population studied, type of prediction model and its variables, type of statistical analysis, type of reference standard, and outcomes. We also separated studies by training, development, and external validation models. The data were obtained in duplicate by V.C. and A.G.-R. and corroborated by E.D.-G. The extraction and analysis were performed by separating healthy populations and type 2 diabetes populations; the results are presented as separate groups.
Two reviewers (V.C. and A.G.-R.) independently assessed the risk of bias with Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2), guided by the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. We used the software tool RevMan 5.4 (Cochrane).
The QUADAS-2 tool evaluated 4 principal domains: 1) patient selection, 2) index test, 3) reference standard, and 4) flow and timing. The applicability concerns were evaluated in 3 domains: 1) patient selection, 2) index test, and 3) reference standard. Each potential bias and concern was graded as high, low, or unclear risk. Risk of bias was evaluated by A.G.-R. and V.C.
Statistical analysis and data synthesis
To synthesize the information, we divided it by population — healthy population and type 2 diabetes population — and looked for homogeneity in the baseline characteristics of the participants of the studies and the possible risk factors. Nevertheless, because of the heterogeneity of metrics and variables used to assess the predictive ability of CKD risk models, we conducted a qualitative synthesis of the full evidence instead of a meta-analysis.
Results
Search results
An electronic search was conducted in May 2021 and updated in January 2022. We identified 2,359 records by searching databases, registers, and other sources; 9 of the studies were identified by doing manual searching, and 1 was from gray literature. From the 2,359 we removed 29 duplicate records. In the screening stage, 1,691 records that did not meet the inclusion criteria were eliminated. Two studies were not retrieved because they were not fully published, leaving 31 studies retrieved for full-text analysis. Of these, 17 were eliminated because they 1) were prediction models for advanced CKD, 2) were about prevalence of CKD, 3) had insufficient data for analysis, or 4) had a sample that mixed type 2 diabetes and type 1 diabetes populations (9). From other methods (reference scanning, manual searching, and gray literature), 7 studies were retrieved. Finally, for the qualitative analysis 21 studies were included: 13 studies (10–22) with prediction models to assess the presence or occurrence of CKD in healthy adults, 7 studies (23–28, and one unpublished paper [A. Raña-Custodio, M. Lajous, E. Denova-Gutiérrez, M. Chávez-Cárdenas, R. Lopez-Ridaura, and G. Danaei, personal communication, 2023]) with prediction model to assess the presence or occurrence of CKD in people with type 2 diabetes, and 1 study including a model for both populations (29). In those studies, we identified 48 different models.
The main characteristics of the risk predictive models for CKD developed in each study are described in Table 1. Fourteen studies were developed by using prospective cohort data, 4 were developed by using cross-sectional data, and 3 were developed by using retrospective cohort data. Of the total studies included, 13 studies’ outcome results were calculated with C statistic, 10 studies with AUC, and 7 studies reported sensitivity and specificity analysis.
Healthy population risk scores
We synthesized information from 14 studies (10–22,29) that developed equations to detect the risk of CKD in healthy populations; 36 different predictive models were identified. Three models used 5 risk factors (11,18,21); the number of factors included in the models ranged from 3 (18) and 146 variables (12). Some of the most common risk factors included for the predictive equation were age, sex, type 2 diabetes (glycated hemoglobin A1c [HbA1c], fasting plasma glucose, or history of diabetes), kidney function (eGFR, proteinuria, or albuminuria), cardiovascular disease (systolic blood pressure, diastolic blood pressure, or history of hypertension), and obesity (waist circumference or body mass index). For the reference standard, all studies used eGFR, with the cutoff established by Kidney Disease Improving Global Outcomes (KDIGO) guidelines (1). Additionally, 4 studies (15,18,20,29) conducted an external validation.
In 7 studies (11,12,16,17,19,20,29) the outcome was calculated with C statistics; in 8 studies (10,13–16,18,21,22), with AUC; 4 studies (11,12,15,21) reported sensitivity and specificity. The range of C statistics was 0.6 to 0.9; the range of AUC was 0.6 to 0.9 (Table 2). We identified for the healthy population 11 models in 7 (10,13–16,18,22) reports predicting CKD above 0.8 AUC.
The prediction score with the highest C statistic was the model derivation (12) that included 146 variables (demographics, clinical, medications, and laboratory test results). The 2 highest AUCs were 1) a model developed by using stepwise analysis (10) that included age, sex, type 2 diabetes (yes/no), hypertension (yes/no), dyslipidemia (yes/no), smoking status (yes/no), cardiovascular disease (yes/no), systolic blood pressure (mm Hg), diastolic blood pressure (mm Hg), total cholesterol (mmol/L), triglycerides (mmol/L), HbA1c (%), and eGFR (mL/min/1.73 m2); and 2) the sex-specific model (22) that included, for male participants, eGFR, HbA1c standard deviation (%), uric acid, uric acid standard deviation, blood urea nitrogen, albumin, and hemoglobin; and for female participants, age, eGFR, triglycerides, HbA1c standard deviation, uric acid, uric acid standard deviation, blood urea nitrogen, albumin, hemoglobin, and age at menarche (when age at menarche was ≥17 years).
The sensitivity range was 50.3 to 89.4; the highest sensitivity value was from the Kwon et al model (15) that incorporated age, sex, anemia, hypertension, type 2 diabetes, cardiovascular disease, and proteinuria. The specificity values range was 0.51 to 97.3; the highest specificity was the derivation model (12) that included 146 variables.
Type 2 diabetes risk scores
We synthesized information from 8 studies (23–29 and 1 unpublished paper [A. Raña-Custodio, M. Lajous, E. Denova-Gutiérrez, M. Chávez-Cárdenas, R. Lopez-Ridaura, and G. Danaei, personal communication, 2023]) that developed risk equations for people with type 2 diabetes, analyzing 11 different models. Most of the predictive models used at least 5 risk factors; the number of factors in the models ranged from 5 (23,24) to 16 variables (Raña-Custodio et al, personal communication, 2023). Some of the most common risk factors included for the predictive equation were age, sex, eGFR, and HbA1c.
In 6 studies (23–25,28,29, and unpublished study), outcome accuracy was calculated with C statistics; in 2 studies, with AUC (26,27); and 2 reported sensitivity and specificity (Table 2). The range of C statistics was 0.5 to 0.8; the highest AUC was 0.83. The highest C statistic was from the external validation of the diabetic model (29); this model included age, sex, race, ethnicity, eGFR, history of cardiovascular disease, ever smoker, hypertension, body mass index, albuminuria, diabetes medications (insulin vs only oral medications vs none), and HbA1c. The range of sensitivity was 64.5 to 75.6; the highest sensitivity was also the external validation of diabetic model. The specificity range was 46.5 to 72.3; the highest specificity was the model developed test data set (26).
Methodologic quality of included studies
Of all studies included in the synthesis, in the patient selection domain 81% had low risk of bias and 76% had low applicability concern (Figure 2). Dunkler et al had unclear concern of bias because the sample included people receiving pharmacologic therapy (24). Hippisley-Cox and Coupland had high applicability concern because the study population had moderate CKD, recorded by kidney transplants and record of kidney dialysis (13). In the index test domain, 86% had low risk of bias and 100% had low applicability concern. In the reference standard domain, 71% had low risk of bias; Saranburut et al used an outcome from a modification of the KDIGO definition (18). Also in the reference standard domain, 90% had low applicability concern. For the flow and timing domain, 95% had low risk of bias.
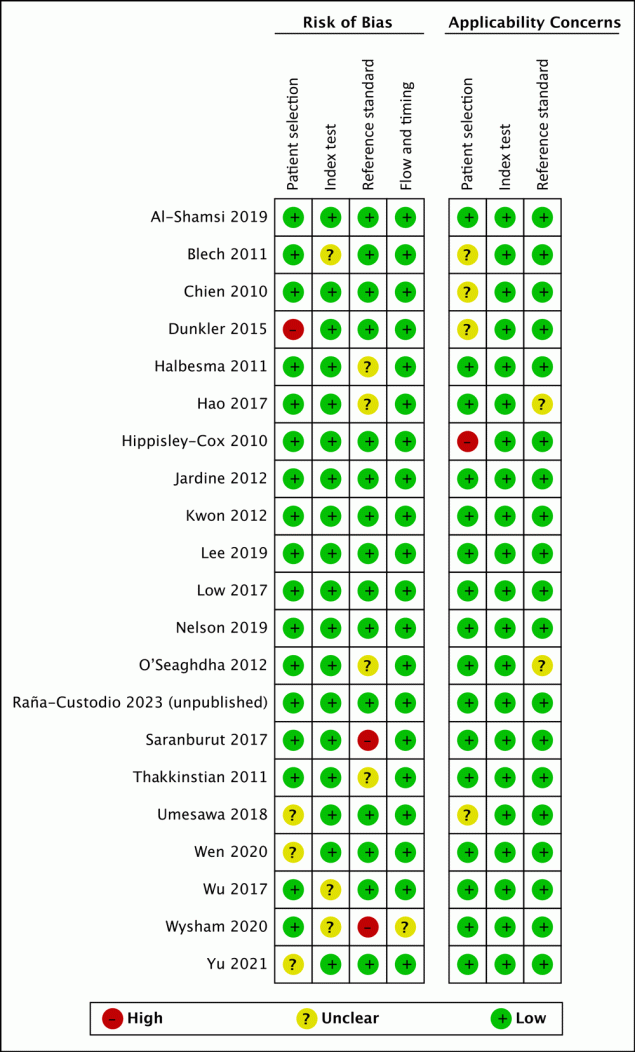
![]()
Figure 2.
Methodologic quality summary and graph for analysis of studies of chronic kidney disease (CKD) in healthy adults and adults living with type 2 diabetes. [A tabular description of this figure is available.]
Discussion
The aim of this systematic review was to identify the existing prediction scores and their diagnostic accuracy for detecting CKD. Thus, we identified 48 different predictive models in 21 total studies of healthy people and people with type 2 diabetes. For healthy populations, we analyzed 14 studies presenting 36 predictive scores for CKD and a wide range (4 to 146) of variables considered by each author. Populations with type 2 diabetes were summarized in 8 studies presenting 15 different models with a range of 4 to 16 variables.
Evaluating the accuracy of these models is a cornerstone to find the best but also reachable way to predict the risk of CKD. In our study, we identified for the healthy population 11 models predicting CKD above 0.8 AUC, considered as good discriminatory performance (30).
This review discords with another review (31). However, by using techniques with a specific tool (QUADAS-2), there are predictive models with good accuracy and quality. For example, Al-Shamsi et al (10) presented a stepwise model for a healthy population with an AUC of 0.9 (95% CI, 0.8–0.9) using variables that are simple and reliable in primary care (eGFR, diabetes, cholesterol, and HbA1c), with low risk of bias and low applicability concern. Also, Yu et al (22) presented a sex-specific model with AUC over 0.9 for both sexes, with low risk of bias and low applicability concern.
For the population with type 2 diabetes, Low et al (26), with 0.8 AUC, 75.6 sensitivity, and 72.3 specificity, had the highest accuracy, considered as good discriminatory performance. This risk score includes variables log albumin-to-creatinine ratio, systolic blood pressure, HbA1c, eGFR, low-density lipoprotein cholesterol, and age, with low risk of bias and applicability concern. The presence of type 2 diabetes is one of the main risk factors for developing CKD; identifying the population at higher risk is vital for public health. In both populations, the risk models with highest accuracy had HbA1c and eGFR variables in common. Chadban et al recognized that blood glucose plays a significant role in the development of CKD (32), and that is shown in the predictive model that includes a variable related to blood glucose.
These models present a wide heterogeneity between the variables included, similar to findings in other reviews (7,31). Regardless, the heterogeneity found between these predictive equations had common variables: age, hypertension-related variables (systolic blood pressure or diastolic blood pressure), body mass index, and diabetes-related variables (history of diabetes, HbA1c, glucose). In agreement with other authors (7), we found that using predictive models with feasible variables in primary care could help professionals from this level of health care alert the population at risk. Also, looking through these variables gave us a chance to look at prevention therapies that control the progression of these variables, as reflected in the progression of CKD. CKD risk predictive models should be applied mainly in populations with risk factors for CKD susceptibility, initiation, or progression (33).
To our knowledge, this is the first systematic review of risk prediction models for CKD that looked at a risk of bias with a validated tool for diagnostic accuracy such as QUADAS-2 to test studies. Conducting a risk of bias analysis is important in a systematic review, because after the accuracy of the studies is identified, the methodologic quality plays an important role for future research and for the populations affected. Also, this review presents results for general populations and for populations with type 2 diabetes that are at higher risk for CKD.
As a limitation, this review did not identify risk models with variables comparable between them to conduct a meta-analysis. Therefore, it is not possible to make recommendations for the use of the models in other populations. We suggest that future work validate in different populations the existing scores and obtain comparable data to make recommendations.
To synthetize the existing models in this report, we gave public health researchers and clinicians a wide view of existing models. From the models they can choose the one that best applies to their population with regard to their accuracy and their methodologic quality.
Conclusions
We synthesized risk models to detect CKD in healthy and type 2 diabetes patients. Of those, 11 models for healthy populations and 3 for type 2 diabetes patients were identified with good discriminatory performance and methodologic quality. The development of these models, using all those different variables, gives a wide observation of the accuracy and the risk factors. The burden of CKD is increasing in both absolute and relative terms; identifying models that can help to predict the risk of CKD could be the first step to prevent CKD and inform the population. These models are important in primary care settings to help identify people at risk and promptly start prevention or treatment. Some of these models had variables easily obtained at primary care services, improving the accuracy in screening the population at risk and referring patients to a specialist as needed. Finally, these tools need to be externally validated to identify their accuracy in other populations, to provide more information to affected populations regarding public policies about the risk of incident CKD.
Acknowledgments
The authors reported no funding for this study and declared no conflict of interest. No borrowed material or copyrighted surveys, instruments, or tools were used for this article.
Author Information
Corresponding Author: Edgar Denova-Gutiérrez, PhD, Centro de Investigación en Nutrición y Salud, Instituto Nacional de Salud Pública, Av Universidad 655, Cuernavaca, Morelos, Mexico, 62100 (edgar.denova@insp.mx).
Author Affiliations: 1Centro de Investigación en Nutrición y Salud, Instituto Nacional de Salud Pública, Cuernavaca, México. 2Facultad de Medicina, Universidad Juárez Autónoma de Tabasco, Tabasco, México.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3(1):1–150.
- Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the Global Burden of Disease study. Nephrol Dial Transplant 2017;32(suppl 2):ii121–8. PubMed doi:10.1093/ndt/gfw330
- Deng Y, Li N, Wu Y, Wang M, Yang S, Zheng Y, et al. Global, regional, and national burden of diabetes-related chronic kidney disease from 1990 to 2019. Front Endocrinol (Lausanne) 2021;12(July):672350. PubMed doi:10.3389/fendo.2021.672350
- Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al; GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2020;395(10225):709–33. PubMed doi:10.1016/S0140-6736(20)30045-3
- Akbari A, Grimshaw J, Stacey D, Hogg W, Ramsay T, Cheng-Fitzpatrick M, et al. Change in appropriate referrals to nephrologists after the introduction of automatic reporting of the estimated glomerular filtration rate. CMAJ 2012;184(5):E269–76. PubMed doi:10.1503/cmaj.110678
- Shlipak MG, Tummalapalli SL, Boulware LE, Grams ME, Ix JH, Jha V, et al; Conference Participants. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2021;99(1):34–47. PubMed doi:10.1016/j.kint.2020.10.012
- Echouffo-Tcheugui JB, Kengne AP. Risk models to predict chronic kidney disease and its progression: a systematic review. PLoS Med 2012;9(11):e1001344. PubMed doi:10.1371/journal.pmed.1001344
- Saputro SA, Pattanateepapon A, Pattanaprateep O, Aekplakorn W, McKay GJ, Attia J, et al. External validation of prognostic models for chronic kidney disease among type 2 diabetes. J Nephrol 2022;35(6):1637–53. PubMed doi:10.1007/s40620-021-01220-w
- Tuntayothin W, Kerr SJ, Boonyakrai C, Udomkarnjananun S, Chukaew S, Sakulbumrungsil R. Development and validation of a chronic kidney disease prediction model for type 2 diabetes mellitus in Thailand. Value Health Reg Issues 2021;24:157–66. PubMed doi:10.1016/j.vhri.2020.10.006
- Al-Shamsi S, Oulhaj A, Regmi D, Govender RD. Use of estimated glomerular filtration rate to predict incident chronic kidney disease in patients at risk of cardiovascular disease: a retrospective study. BMC Nephrol 2019;20(1):325. PubMed doi:10.1186/s12882-019-1494-8
- Chien KL, Lin HJ, Lee BC, Hsu HC, Lee YT, Chen MF. A prediction model for the risk of incident chronic kidney disease. Am J Med 2010;123(9):836–46.e2. PubMed doi:10.1016/j.amjmed.2010.05.010
- Hao S, Fu T, Wu Q, Jin B, Zhu C, Hu Z, et al. Estimating one-year risk of incident chronic kidney disease: retrospective development and validation study using electronic medical record data from the state of Maine. JMIR Med Inform 2017;5(3):e21. PubMed doi:10.2196/medinform.7954
- Hippisley-Cox J, Coupland C. Predicting the risk of chronic kidney disease in men and women in England and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam Pract 2010;11(1):49. PubMed doi:10.1186/1471-2296-11-49
- Halbesma N, Jansen DF, Heymans MW, Stolk RP, de Jong PE, Gansevoort RT; PREVEND Study Group. Development and validation of a general population renal risk score. Clin J Am Soc Nephrol 2011;6(7):1731–8. http://cjasn.asnjournals.org/content/6/7/1731 PubMed doi:10.2215/CJN.08590910
- Kwon KS, Bang H, Bomback AS, Koh DH, Yum JH, Lee JH, et al. A simple prediction score for kidney disease in the Korean population. Nephrology (Carlton) 2012;17(3):278–84. PubMed doi:10.1111/j.1440-1797.2011.01552.x
- Lee C, Yun HR, Joo YS, Lee S, Kim J, Nam KH, et al. Framingham risk score and risk of incident chronic kidney disease: a community-based prospective cohort study. Kidney Res Clin Pract 2019;38(1):49–59. PubMed doi:10.23876/j.krcp.18.0118
- O’Seaghdha CM, Lyass A, Massaro JM, Meigs JB, Coresh J, D’Agostino RB Sr, et al. A risk score for chronic kidney disease in the general population. Am J Med 2012;125(3):270–77. PubMed doi:10.1016/j.amjmed.2011.09.009
- Saranburut K, Vathesatogkit P, Thongmung N, Chittamma A, Vanavanan S, Tangstheanphan T, et al. Risk scores to predict decreased glomerular filtration rate at 10 years in an Asian general population. BMC Nephrol 2017;18(1):240. PubMed doi:10.1186/s12882-017-0653-z
- Thakkinstian A, Ingsathit A, Chaiprasert A, Rattanasiri S, Sangthawan P, Gojaseni P, et al. A simplified clinical prediction score of chronic kidney disease: a cross-sectional-survey study. BMC Nephrol 2011;12(1):45. PubMed doi:10.1186/1471-2369-12-45
- Umesawa M, Sairenchi T, Haruyama Y, Nagao M, Yamagishi K, Irie F, et al. Validity of a risk prediction equation for CKD after 10 years of follow-up in a Japanese population: the Ibaraki Prefectural Health Study. Am J Kidney Dis 2018;71(6):842–50. PubMed doi:10.1053/j.ajkd.2017.09.013
- Wen J, Hao J, Zhang Y, Cao K, Zhang X, Li J, et al. Risk scores for predicting incident chronic kidney disease among rural Chinese people: a village-based cohort study. BMC Nephrol 2020;21(1):120. PubMed doi:10.1186/s12882-020-01787-9
- Yu Y, Zhao Q, Jiang Y, Wang N, Liu X, Qiu Y, et al. Prediction models and nomograms of 3-year risk of chronic kidney disease in China: a study from the Shanghai Suburban Adult Cohort and Biobank (2016–2020). Ann Transl Med 2021;9(22):1690. PubMed doi:10.21037/atm-21-5647
- Blech I, Katzenellenbogen M, Katzenellenbogen A, Wainstein J, Rubinstein A, Harman-Boehm I, et al. Predicting diabetic nephropathy using a multifactorial genetic model. PLoS One 2011;6(4):e18743. PubMed doi:10.1371/journal.pone.0018743
- Dunkler D, Gao P, Lee SF, Heinze G, Clase CM, Tobe S, et al; ONTARGET and ORIGIN Investigators. Risk prediction for early CKD in type 2 diabetes. Clin J Am Soc Nephrol 2015;10(8):1371–9. PubMed doi:10.2215/CJN.10321014
- Jardine MJ, Hata J, Woodward M, Perkovic V, Ninomiya T, Arima H, et al; ADVANCE Collaborative Group. Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis 2012;60(5):770–8. PubMed doi:10.1053/j.ajkd.2012.04.025
- Low S, Lim SC, Zhang X, Zhou S, Yeoh LY, Liu YL, et al. Development and validation of a predictive model for chronic kidney disease progression in type 2 diabetes mellitus based on a 13-year study in Singapore. Diabetes Res Clin Pract 2017;123:49–54. PubMed doi:10.1016/j.diabres.2016.11.008
- Wu M, Lu J, Zhang L, Liu F, Chen S, Han Y, et al. A non-laboratory-based risk score for predicting diabetic kidney disease in Chinese patients with type 2 diabetes. Oncotarget 2017;8(60):102550–8. PubMed doi:10.18632/oncotarget.21684
- Wysham CH, Gauthier-Loiselle M, Bailey RA, Manceur AM, Lefebvre P, Greenberg M, et al. Development of risk models for major adverse chronic renal outcomes among patients with type 2 diabetes mellitus using insurance claims: a retrospective observational study. Curr Med Res Opin 2020;36(2):219–27. PubMed doi:10.1080/03007995.2019.1682981
- Nelson RG, Grams ME, Ballew SH, Sang Y, Azizi F, Chadban SJ, et al; CKD Prognosis Consortium. Development of risk prediction equations for incident chronic kidney disease. JAMA 2019;322(21):2104–14. PubMed doi:10.1001/jama.2019.17379
- Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA 2017;318(14):1377–84. PubMed doi:10.1001/jama.2017.12126
- Collins GS, Omar O, Shanyinde M, Yu LM. A systematic review finds prediction models for chronic kidney disease were poorly reported and often developed using inappropriate methods. J Clin Epidemiol 2013;66(3):268–77. PubMed doi:10.1016/j.jclinepi.2012.06.020
- Chadban S, Howell M, Twigg S, Thomas M, Jerums G, Cass A, et al; CARI. The CARI guidelines. Prevention and management of chronic kidney disease in type 2 diabetes. Nephrology (Carlton) 2010;15(suppl 1):S162–94. PubMed doi:10.1111/j.1440-1797.2010.01240.x
- Colli VA, González-Rocha A, Canales D, Hernández-Alcáraz C, Pedroza A, Pérez-Chan M, et al. Chronic kidney disease risk prediction scores assessment and development in Mexican adult population. Front Med (Lausanne) 2022;9:903090. PubMed doi:10.3389/fmed.2022.903090
Tables
| Author and year | Population, total n; outcomes, n (age) | Study design | Type of statistical analysis | Model identification | Variables included | Accuracy predictor |
|---|---|---|---|---|---|---|
| Healthy adults | ||||||
| Al-Shamsi et al (10), 2019 | 622; 71 (52.4 y) | Retrospective cohort | Fine and gray regression | Full model | Age, sex, diabetes mellitus, hypertension, dyslipidemia, smoking, cardiovascular disease, systolic blood pressure, diastolic blood pressure, total cholesterol, triglycerides, HbA1c, eGFR | AUC |
| Stepwise model | eGFR, diabetes, cholesterol, HbA1c | |||||
| Chien et al (11), 2010 | 5,168; 190 (51.2 y) | Prospective cohort | Cox proportional hazards regression | Clinical model | Age, BMI, diastolic blood pressure (mm Hg), history of type 2 diabetes, history of stroke | C statistic, sensitivity, specificity |
| Biochemical model | Age, diastolic blood pressure, history of stroke, uric acid, postprandial glucose, HbA1c, urine protein ≥100 mg/dL | |||||
| Hao et al (12), 2017 | 1,310,363; 7,448 (NR) | Retrospective cohort | Multivariable logistic regression | Model derivationa | 146 clinical variables including demographics, diagnosis of type 2 diabetes, medications, laboratory test results, and resource utilization | C statistic, sensitivity, specificity |
| 1,430,772; 8,299 (NR) | Model validation | |||||
| Hippisley-Cox and Coupland (13), 2010 | 1,591,884; 23,786 (35–74 y) | Prospective cohort | Cox proportional hazards regression | Final model | Age, ethnicity, deprivation, smoking, BMI, systolic blood pressure, type 2 diabetes, rheumatoid arthritis, cardiovascular disease, treated hypertension, congestive cardiac failure; peripheral vascular disease, NSAID use, family history of kidney disease, systemic lupus erythematosus (in women), and kidney stones (in women) | AUC |
| Halbesma et al (14), 2011 | 6,809; 272 (28–75 y) | Prospective cohort | Multivariable logistic regression | Final model | Age, urinary albumin excretion, systolic blood pressure, C-reactive protein, known hypertension | AUC |
| Kwon et al (15), 2012 | 2,921; NR (≥19 y) | Cross-sectional | Multivariable logistic regression | NR | Age, sex, anemia, hypertension, diabetes, cardiovascular disease, and proteinuria | AUC, sensitivity, specificity |
| External validation 8,166; NR (≥30 y) | Age, sex, anemia, hypertension, diabetes, cardiovascular disease, and proteinuria | |||||
| Lee et al (16), 2019 | 9,080; 734 (51.8 y) | Prospective cohort | Cox proportional hazards regression | Model 1 | Sex, BMI, education level, income, fasting plasma glucose, serum albumin | AUC, C statistic |
| Model 2 | Sex, BMI, education level, income, fasting plasma glucose, serum albumin, Framingham risk score | |||||
| Model 3 | Sex, BMI, education level, income, fasting plasma glucose, serum albumin, eGFR, proteinuria | |||||
| Model 4 | Sex, BMI, education level, income, fasting glucose, serum albumin, eGFR, proteinuria, Framingham risk score | |||||
| Nelson et al (29), 2019 | 5,222,711; 974,502 (NR) | Cross-sectional | Multivariable logistic regression | Primary model | Age, sex, race, ethnicity, eGFR, history of cardiovascular disease, ever smoker, hypertension, BMI, albuminuria | C statistic |
| O’Seaghdha et al (17), 2012 | 2,490; 229 (45–64 y) | Prospective cohort | Multivariable logistic regression | Model 1: clinical model | Age, type 2 diabetes, hypertension | C statistic |
| Model 2: clinical model and baseline eGFR | Age, diabetes mellitus, hypertension, baseline eGFR | |||||
| Model 3: model 2 plus measure of proteinuria | Age, diabetes mellitus, hypertension, baseline eGFR, quantitative albuminuria (urine ACR or dipstick proteinuria) | |||||
| Saranburut et al (18), 2017 | 3,186; 271 (25–54 y) | Prospective cohort | Multivariable logistic regression | Model 1 (clinical)a | Age, sex, history of diabetes, systolic blood pressure, waist circumference | AUC |
| Model 1aa | Substitution of waist circumference with overweight (BMI ≥25) | |||||
| Model 1ba | Substitution of hypertension for systolic blood pressure | |||||
| Model 2 (clinical plus limited laboratory tests)a | Age, sex, systolic blood pressure, diabetic mellitus, GFR category | |||||
| Model 2aa | Substitution of systolic blood pressure with hypertension | |||||
| Model 3 (clinical plus full laboratory tests)a | Age, sex, systolic blood pressure, diabetic mellitus, GFR, uric acid, hemoglobin | |||||
| Model 3aa | Substitution of hypertension for systolic blood pressure | |||||
| Model 1 (clinical) | Age, sex, history of diabetes, systolic blood pressure, waist circumference or BMI | |||||
| Model 2 (clinical plus limited laboratory tests) | Age, sex, systolic blood pressure, diabetic mellitus, GFR category | |||||
| External validation 1,395 (35–54 y) | Model 1 (clinical) | Age, sex, history of diabetes, systolic blood pressure, waist circumference | ||||
| Model 2 (clinical plus limited laboratory tests) | Age, sex, systolic blood pressure, diabetic mellitus, GFR category | |||||
| Thakkinstian et al (19), 2011 | 3,459; 626 (≥18 y) | Cross-sectional | Multivariable logistic regression | Model 1 | Age, diabetes, hypertension, history of kidney stones | C statistic |
| Umesawa et al (20), 2018 | 58,855; 7,500 (40–74 y) | Prospective cohort | Multivariable logistic regression | Simple risk prediction | Age, eGFR, proteinuria, hematuria | C statistic |
| Full risk prediction | Age, eGFR, proteinuria, hematuria, BMI, systolic blood pressure, medication for hypertension, glucose tolerance, medication for diabetes mellitus, smoking and alcohol intake | |||||
| External validation 76,152; 8,964 (40–74 y) | Simple risk prediction | Age, eGFR, proteinuria, and hematuria | ||||
| Full risk prediction | Age, eGFR, proteinuria, hematuria, BMI, systolic blood pressure, medication for hypertension, glucose tolerance, medication for diabetes mellitus, smoking and alcohol intake | |||||
| Wen et al (21), 2020 | 3,266; 590 (NR) | Prospective cohort | Multivariable logistic regression | Training: simple clinical modela | Sex, waist circumference, systolic blood pressure, diabetes mellitus, education | AUC, sensitivity, specificity |
| Best fit modela | Sex, systolic blood pressure, diabetes mellitus, education, triglyceride, urine ACR, C-reactive protein | |||||
| Validation: simple clinical model | Sex, waist circumference, systolic blood pressure, diabetes mellitus, education | |||||
| Best fit model | Sex, systolic blood pressure, diabetes, education, triglyceride, urine ACR, C-reactive protein | |||||
| Yu et al (22), 2021 | 10,049 total; male: 4,117; 157 (NR) | Prospective cohort | Cox proportional hazards regression | Sex-specific CKD male model | eGFR, HbA1c standard deviation, uric acid, uric acid standard deviation, blood urea nitrogen, albumin, hemoglobin | AUC |
| 10,049 total; female: 5,932; 270 (NR) | Sex-specific CKD female model | Age, eGFR, triglycerides, HbA1c standard deviation, uric acid, uric acid standard deviation, blood urea nitrogen, albumin, hemoglobin, age at menarche (if ≥17 years) | ||||
| Adults with type 2 diabetes | ||||||
| Blech et al (23), 2011 | 1,274; 556 (62.6 y) | Cross-sectional | Multivariable logistic regression | Score 1 | Age, duration of diabetes, diabetes type, sex, ethnicity | C statistic, sensitivity, specificity |
| Score 2 | 5 single-nucleotide polymorphisms in 5 genes (HSPG2, NOS3, ADIPOR2, AGER, CCL5), age, duration of diabetes, diabetes type, sex, ethnicity | |||||
| Dunkler et al (24), 2015 | 6,766; 1,079 (≥55 y) | Prospective cohort | Multivariable logistic regression | Laboratory model | Urine ACR, eGFR, albuminuria stage (normo- or microalbuminuria), sex, age | C statistic, sensitivity, specificity |
| External validation 8,300 (≥55 y) | Clinical model | Delta–urine albumin-creatinine ratio to progression, eGFR, albuminuria stage, sex, age, race (White, Asian, other), diabetes duration (years, log transformed), fasting LDL (mg/dL), glucose (mg/dl), waist circumference (cm), comorbidities major atherosclerotic cardiac events (myocardial infarction, stable or unstable angina, coronary artery bypass grafting, or percutaneous interventions, including angioplasty, stenting, atherectomy), laser therapy for diabetic retinopathy, peripheral artery disease (peripheral arterial angioplasty, limb or foot amputation), stroke or transient ischemic attack, number of antihypertensive drugs prescribed | ||||
| Jardine et al (25), 2012 | 7,377; 2,715 (NR) | Prospective cohort | Cox proportional hazards regression | eGFR ACR model | eGFR, ACR | C statistic |
| Final risk prediction model | Ethnicity, eGFR, ACR, systolic blood pressure, hypertension treatment, HbA1c level, diabetic retinopathy, waist circumference | |||||
| External validation 11,140 (NR) | eGFR, urinary ACR, systolic blood pressure, HbA1c level, diabetic retinopathy, blood pressure–lowering treatment at baseline, Asian ethnicity, waist circumference | |||||
| Low et al (26), 2017 | 1,582; 679 (NR) | Prospective cohort | Multivariable logistic regression | Training data seta | Log urinary ACR (mg/g), systolic blood pressure (per 10 mm Hg), HbA1c, eGFR, (per 5 ml/min/1.73m2), LDL cholesterol (mmol/L), age (per 10 years increase) | AUC, sensitivity, specificity |
| Test data set | Log urinary ACR, systolic blood pressure, HbA1c, eGFR, LDL cholesterol, age (per 10 years increase) | |||||
| Nelson et al (29), 2019 | 5,222,711; 974,502 (NR) | Cross-sectional | Multivariable logistic regression | Diabetic model | Age, sex, race, ethnicity, eGFR, history of cardiovascular disease, ever smoker, hypertension, BMI, albuminuria, diabetes medications (insulin vs only oral medications vs none), HbA1c values, and the interaction between diabetes medications and HbA1c values | C statistic |
| External validation | 2,253,540; 367,159 (NR) | External validation model | ||||
| Raña-Custodio et al (unpublished data)b | 18,148; 1,617 (60.5 y) | Prospective cohort | Multivariable logistic regression | Office equation | Sex, age, BMI, current tobacco smoking and alcohol intake, evolution of diabetes mellitus, current diabetes mellitus treatment scheme (insulin, oral hypoglycemics, or both), prevalent microvascular complication of type 2 diabetes (retinopathy, diabetic foot, neuropathy, or stroke), square of age and female current smoker | C statistic |
| Laboratory risk score | Sex, age, BMI, current tobacco smoking and alcohol intake, family history of CKD (defined as any prevalent history of CKD among any first-degree relative), history of hypertension, evolution of diabetes mellitus, current diabetes mellitus treatment scheme (insulin, oral hypoglycemics, or both), microvascular complication of type 2 diabetes (retinopathy, diabetic foot, neuropathy, or stroke), fasting plasma glucose, HbA1c, serum creatinine (isotope dilution mass spectrometry), eGFR (Chronic Kidney Disease Epidemiology Collaboration equation), total cholesterol, triglyceride levels | |||||
| Wu et al (27), 2017 | 4,795; 643 (59.3 y) | Prospective cohort | Multivariable logistic regression | Development model | Sex, BMI, systolic blood pressure, duration of diabetes (years) | AUC |
| Wysham et al (28), 2020 | 160,031; 9,973 (NR) | Retrospective cohort | Multivariable logistic regression | DKD | Age, sex, geographic region, insurance type, payer type, adapted diabetes complications severity index, a recorded diagnosis of hypertension, a recorded diagnosis of heart failure, anemia, diabetic nephropathy, CKD stage 1 and 2, time interval with diabetes mellitus | C statistic |
Abbreviations: ACR, albumin-to-creatinine ratio; AUC, area under the receiver operating characteristic curve; BMI, body mass index; DKD, diabetic kidney disease; eGFR, (estimated) glomerular filtration rate; HbA1c, glycated hemoglobin A1c; LDL, low-density lipoprotein; NR, not reported; NSAID, nonsteroidal anti-inflammatory drug.
a Development models.
b A. Raña-Custodio; M. Lajous; E. Denova-Gutiérrez, PhD; M. Chávez-Cárdenas; R. Lopez-Ridaura; G. Danaei, personal communication, 2023.
| Author and year | Model name/stage | AUC (95% CI) | C statistic (95% CI) | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Healthy adults | |||||
| Al-Shamsi et al (10), 2019 | Full model | 0.90 (0.85–0.95) | NR | NR | NR |
| Stepwise model | 0.91 (0.85–0.96) | NR | NR | NR | |
| Chien et al (11), 2010 | Clinical model | NR | 0.76 | 0.76 | 0.66 |
| Biochemical model | NR | 0.76 | 0.88 | 0.51 | |
| Hao et al (12), 2017 | Model derivation | NR | 0.91 | 62.61 (95% CI, 61.50−63.71) | 97.33 (95% CI, 97.30−97.36) |
| Model validation | NR | 0.87 | 50.33 (95% CI, 49.25−51.41) | 96.60 (95% CI, 96.57−96.63) | |
| Hippisley-Cox and Coupland (13), 2010 | Final model (THIN) | Male: 0.87 (0.87–0.88); female: 0.87 (0.87–0.88) | NR | NR | NR |
| (QResearch) | Male: 0.87 (0.87–0.88); female: 0.87 (0.87–0.88) | NR | NR | NR | |
| Halbesma et al (14), 2011 | Final model | 0.84 (0.82–0.86) | NR | NR | NR |
| Kwon et al (15), 2012 | NR | 0.87 (0.84–0.89) | NR | 89.4 (95% CI, 84.4–93.2) | 70.6 (95% CI, 68.90–72.30) |
| External validation | 0.78 (0.76–0.80) | NR | NR | NR | |
| Lee et al (16), 2019 | Model 1 | 0.63 (0.61–0.65) | 0.65 (0.63–0.67) | NR | NR |
| Model 2 | 0.69 (0.68–0.72) | 0.72 (0.70–0.74) | NR | NR | |
| Model 3 | 0.79 (0.78–0.81) | 0.81 (0.80–0.83) | NR | NR | |
| Model 4 | 0.81 (0.80–0.83) | 0.83 (0.82–0.85) | NR | NR | |
| Nelson et al (29), 2019 | Primary model | NR | 0.87 (0.82–0.90) | NR | NR |
| External validation | NR | 0.84 (0.83–0.87) | NR | NR | |
| O’Seaghdha et al (17), 2012 | Model 1: clinical model | NR | 0.79 | NR | NR |
| Model 2: clinical model and baseline eGFR | NR | 0.81 | NR | NR | |
| Model 3: model 2 plus measure of proteinuria | NR | 0.81 | NR | NR | |
| Saranburut et al (18), 2017 | Model 1 (clinical) | 0.72 (0.69–0.75) | NR | NR | NR |
| Model 1a | 0.72 (0.69–0.75) | NR | NR | NR | |
| Model 1b | 0.71 (0.68–0.74) | NR | NR | NR | |
| Model 2 (clinical and limited laboratory tests) | 0.79 (0.76–0.82) | NR | NR | NR | |
| Model 2a | 0.78 (0.76–0.81) | NR | NR | NR | |
| Model 3 (clinical and full laboratory tests) | 0.80 (0.77–0.82) | NR | NR | NR | |
| Model 3a | 0.79 (0.76–0.82) | NR | NR | NR | |
| Model 1 (clinical) | 0.71 (0.68–0.74) | NR | NR | NR | |
| Model 2 (clinical plus limited laboratory tests) | 0.75 (0.72–0.78) | NR | NR | NR | |
| External validation: model 1 (clinical) | 0.66 (0.55–0.78) | NR | NR | NR | |
| Model 2 (clinical and limited laboratory tests) | 0.88 (0.80–0.95) | NR | NR | NR | |
| Thakkinstian et al (19), 2011 | Model 1 | NR | Derivative 0.77 | NR | NR |
| Model 2 | NR | Validated 0.74 | NR | NR | |
| Umesawa et al (20), 2018 | Simple risk prediction | NR | Male: 0.82; female: 0.82 | NR | NR |
| Full risk prediction | NR | Male: 0.82; female: 0.82 | NR | NR | |
| External validation: simple risk prediction | NR | Male: 0.82; female: 0.81 | NR | NR | |
| Full risk prediction | NR | Male: 0.83; female: 0.81 | NR | NR | |
| Wen et al (21), 2020 | Training: simple clinical model | 0.71 (0.68–0.74) | NR | NR | NR |
| Training: best-fit model | 0.72 (0.69–0.75) | NR | NR | NR | |
| Validation: simple clinical model | 0.71 (0.68–0.74) | NR | 70.49 (95% CI, 63.30–77.00) | 65.14 (61.90–68.30) | |
| Validation: best-fit model | 0.72 (0.69–0.74) | NR | 56.83 (95% CI, 49.30–64.10) | 76.61 (73.70–79.40) | |
| Yu et al (22), 2021 | Sex-specific CKD male model | 0.93 (0.90–0.96) | NR | NR | NR |
| Sex-specific CKD female model | 0.95 (0.93–0.97) | NR | NR | NR | |
| Adults living with type 2 diabetes | |||||
| Blech et al (23), 2011 | Score 1 | NR | 0.56 | 64.55 | 46.54 |
| Dunkler et al (24), 2015 | Laboratory model | NR | 0.67 | NR | NR |
| Clinical model | NR | 0.69 | NR | NR | |
| External validation | NR | 0.69 | NR | NR | |
| Jardine et al (25), 2012 | eGFR plus albumin-to-creatinine ratio model | NR | 0.62 (0.61–0.64) | NR | NR |
| Final risk prediction model | NR | 0.64 (0.63–0.65) | NR | NR | |
| External validation | NR | 0.64 | NR | NR | |
| External validation | NR | 0.62 | NR | NR | |
| Low et al (26), 2017 | Training data set | 0.80 (0.77–0.83) | NR | 71.4 | 72.2 |
| Test data set | 0.83 (0.79–0.87) | NR | 75.6 | 72.3 | |
| Nelson et al (29), 2019 | Diabetic model | NR | 0.80 (0.79–0.83) | NR | NR |
| External validation | NR | 0.81 (0.80–0.82) | NR | NR | |
| Raña-Custodio et al (unpublished data)a | Office equation | NR | 0.67 | NR | NR |
| Laboratory risk score | NR | 0.71 | NR | NR | |
| Wu et al (27), 2017 | Development model | 0.71 (0.69–0.73) | NR | NR | NR |
| Wysham et al (28), 2020 | DKD model | NR | 0.70 | NR | NR |
Abbreviations: AUC, area under the receiver operating characteristic curve; CKD, chronic kidney disease; DKD, diabetic kidney disease; NR, not reported.
a A. Raña-Custodio, M. Lajous, E. Denova-Gutiérrez, PhD, M. Chávez-Cárdenas, R. Lopez-Ridaura, G. Danaei, personal communication, 2023.
Appendix
| Database | Search strategy |
|---|---|
| Cochrane Library | (chronic kidney insufficiency) OR (Chronic kidney disease) AND (Predictive models) AND adults |
| Medline/PubMed | (((“risk models”[Title/Abstract] OR “predictive models”[Title/Abstract] OR “Algorithm”[Title/Abstract])) AND ((“chronic kidney disease”[Title/Abstract]) OR (chronic renal insufficiency[MeSH Terms]))) AND (2011:2021[pdat]) |
| Embase | (‘chronic kidney failure’ AND ‘risk assessment’ OR ‘predictive accuracy’) AND ‘normal human’ |
| Latin American and Caribbean Health Sciences Literature (LILACS) | (predictive models) OR (clinical decision rules) AND (chronic renal insufficiency) OR (chronic kidney disease) AND (db:(“LILACS” OR “IBECS” OR “CUMED” OR “BINACIS” OR “BDENF” OR “MULTIMEDIA” OR “PREPRINT-MEDRXIV” OR “BBO” OR “BIGG”)) |
| Cumulative Index to Nursing and Allied Health Literature (CINAHL) | ((predictive models) OR (clinical decision rules)) AND (chronic renal insufficiency or chronic kidney disease or chronic kidney insufficiency or chronic renal disease) |
| PsycInfo | ((predictive models) OR (clinical decision rules)) AND (chronic renal insufficiency or chronic kidney disease or chronic kidney insufficiency or chronic renal disease) |
| Trip Database | (title:adults)(title:(predictive models) OR (clinical decision rules) AND (chronic renal insufficiency) OR (chronic kidney disease)) |
| Epistemonikos | (title:((risk models OR predictive models OR Algorithm)) OR abstract:((risk models OR predictive models OR Algorithm))) AND (title:((chronic kidney disease OR chronic renal insufficiency)) OR abstract:((chronic kidney disease OR chronic renal insufficiency))) |
| Health Evidence | [(chronic kidney disease) OR (chronic kidney failure) AND (Risk model) OR predictive] AND Limit: Date = Published from 2010 to 2021 |
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.