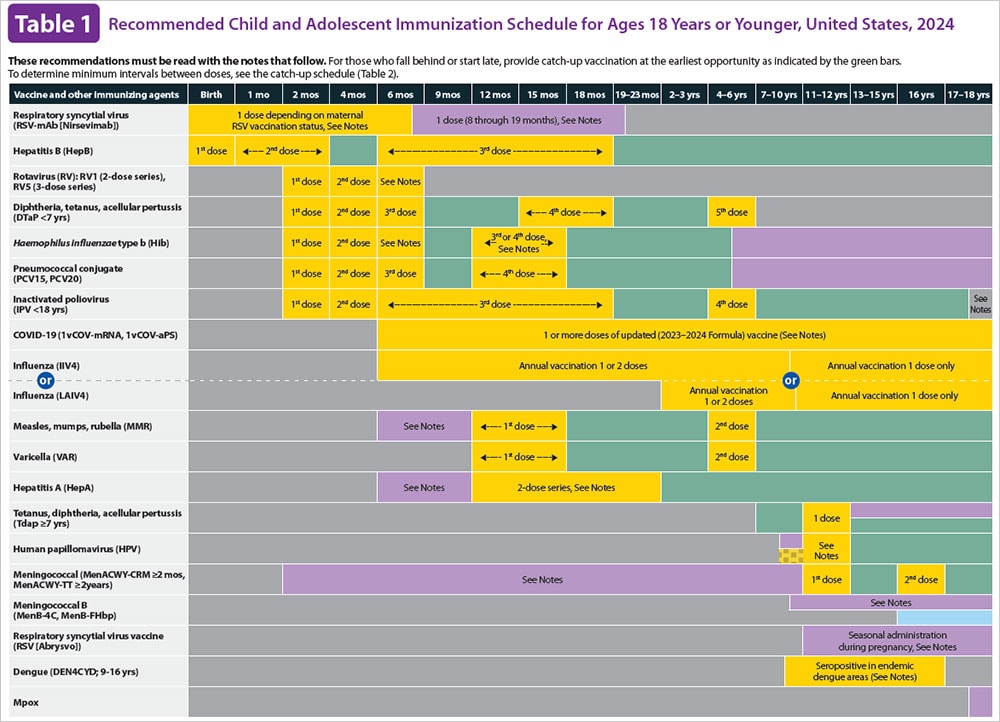
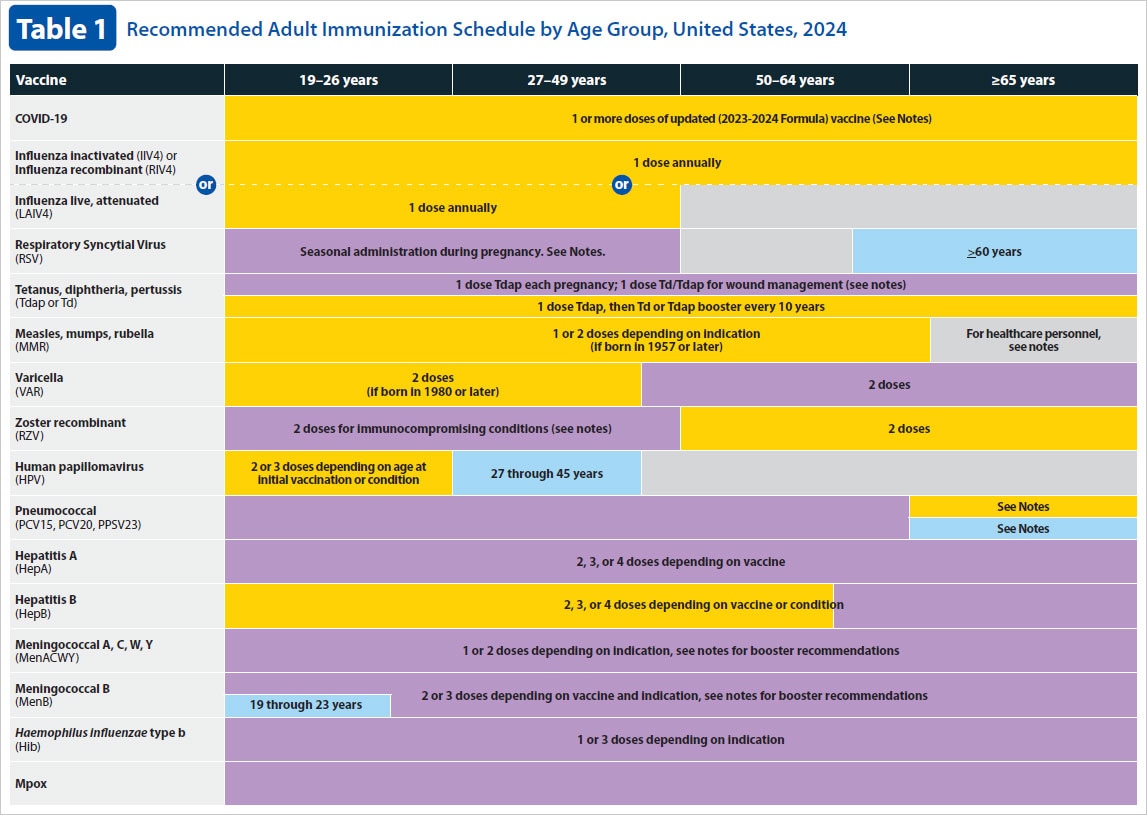
Current VISs
Currently, providers are required by law to provide EUA fact sheets to vaccine recipients or their caregivers for all uses of Novavax and when Moderna or Pfizer vaccines are given to children 6 months through 11 years of age. For recipients who are 12 or older receiving Pfizer or Moderna vaccine, a provider may use the COVID-19 Vaccine Information Statement (VIS).
CDC maintains a current English language VIS for each vaccine. You and your patients can
- View and display the web page
- Download and print the PDF file
- Import the RTF (text) file into an electronic system
- View on a smartphone, tablet or other web-accessible mobile device
- The date, in red, next to each VIS is the most recent version.
- The Interim version is to be used until the final version is available.
See What’s New to learn when the final version should be available.
See FAQs on when to start using a new VIS.
Multi
- Multiple Vaccines (DTaP, Hib, Hepatitis B, PCV, and Polio) interim (7/24/23)
This VIS may be used in place of the individual VISs for DTaP, Hib, Hepatitis B, Polio, and PCV13 when two or more of these vaccines are administered during the same visit. It may be used for infants through children receiving their routine 4-6 year vaccines.
Routine
- COVID-19 (10/17/24)
- Dengue (12/17/21)
- DTaP (Diphtheria, Tetanus, Pertussis) (8/6/21)
- Hepatitis A (10/15/21)
- Hepatitis B (5/12/23) interim
- Hib (Haemophilus Influenzae type b) (8/6/21)
- HPV (Human Papillomavirus) (8/6/21)
- Influenza - Live, Intranasal (8/6/21)
- Influenza - Inactivated (8/6/21)
- Measles/Mumps/Rubella (MMR) (8/6/21)
- Measles/Mumps/Rubella & Varicella (MMRV) (8/6/21)
- Meningococcal ACWY (8/6/21)
- Meningococcal B (8/6/21)
- Pneumococcal Conjugate (PCV) (5/12/23) interim
- Pneumococcal Polysaccharide (PPSV23) (10/30/19)
- Polio (8/6/21)
- Rotavirus (10/15/21)
- Respiratory Syncytial Virus (RSV) Vaccine (10/17/24)
- Tdap (Tetanus, Diphtheria, Pertussis) (8/6/21)
- Td (Tetanus, Diphtheria) (8/6/21)
- Varicella (Chickenpox) (8/6/21)
- Zoster / Shingles (Recombinant) (2/4/22)
Non-routine
- Adenovirus (1/8/20)
Note: Adenovirus vaccine is approved for use only among military personnel. - Anthrax (1/8/20)
- Cholera (10/17/24)
- Ebola (6/30/22)
- Japanese Encephalitis (8/15/19)
- Rabies (6/2/22)
- Smallpox/Monkeypox (JYNNEOS™) (11/14/22)
- Smallpox (ACAM2000®) (12/1/15)
Medical Guide for vaccination with ACAM2000 [6 pages] This medication guide replaces the Smallpox VIS. It is to be used before one receives the vaccination. This guide is not available in other languages. - Tick-borne Encephalitis Vaccine (12/7/23)
- Typhoid (10/30/19)
- Yellow Fever (4/1/20)