At a glance
- The Vaccine Safety Datalink (VSD) is a collaborative project that monitors the safety of vaccines and conducts studies on adverse events (side effects or health problems that occur after vaccination).
- VSD uses electronic health record data from its member sites to assess vaccine safety and detect adverse events in near-real time.
- VSD findings inform U.S. vaccine safety recommendations.
Overview
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC and healthcare organizations across the United States. Established in 1990, the VSD members monitor the safety of vaccines and conduct studies about rare and serious adverse events following immunization.
VSD is made up of 13 member sites:
- 11 full participant sites provide electronic health record (EHR) data shared under a common data model with a standard data dictionary.
- Two sites provide subject matter expertise.
VSD members across the United States
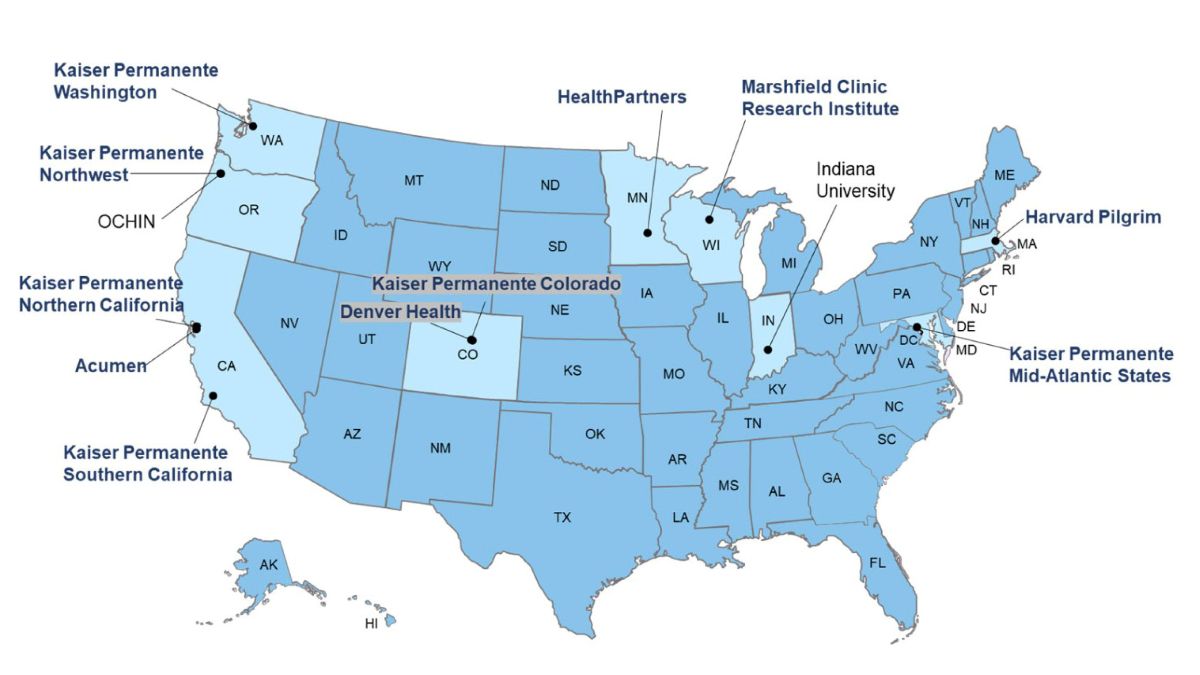
Full participant member sites
Subject matter expert only sites
Objectives
- Conduct research on important vaccine safety questions in large populations.
- Conduct vaccine safety studies from questions or concerns in the medical literature or from other vaccine safety systems such as the Vaccine Adverse Event Reporting System (VAERS).
- Monitor possible rare and serious adverse events when the FDA licenses new vaccines or when the CDC Advisory Committee on Immunization Practices (ACIP) makes new vaccine recommendations. ACIP makes vaccine safety recommendations for the United States.
- Provide timely information to ACIP.
VSD data
To accomplish these objectives, VSD reviews:
- EHR data from member sites that are healthcare organizations, including:
- The type of vaccine given
- The date of vaccination
- Other vaccinations given on the same day
- Medical illnesses diagnosed during any visit to the healthcare facility
How the data are used
Evaluating adverse events in near-real time
Researchers analyze EHR data from member sites every week to determine if the rates of specific adverse events of special interest (AESI) following a specific vaccine are higher than those in a comparison group.
- If the rate is higher in the vaccinated group beyond a certain limit, the vaccine may be associated with an adverse event. If this occurs, CDC conducts follow-up analyses.
- VSD publishes important safety information about many vaccines — including COVID-19, HPV, influenza and more — using RCA. 12345678910111213
Evaluating safety of vaccines in pregnancy
- VSD has developed algorithms that do the following:
- Identify women who are pregnant
- Determine the start and end dates of their pregnancies
- Link medical records for pregnant women and their infants
This helps ensure the desired study participants meet the criteria for inclusion. With the appropriate study population defined, VSD can then conduct vaccine safety studies on this population and their children.
Types of research methods
- Maximized sequential probability ratio test (MaxSPRT, CMaxSPRT)
- Group sequential analysis
- Case-centered analysis
- Sequential concurrent comparator analysis
- Tree-scan analysis
- Hanson, K. E., Goddard, K., Lewis, N., Fireman, B., Myers, T. R., Bakshi, N., Weintraub, E., Donahue, J. G., Nelson, J. C., Xu, S., Glanz, J. M., Williams, J. T. B., Alpern, J. D., & Klein, N. P. (2022). Incidence of Guillain-Barré Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. JAMA network open, 5(4), e228879. https://doi.org/10.1001/jamanetworkopen.2022.8879
- Goddard, K., Lewis, N., Fireman, B., Weintraub, E., Shimabukuro, T., Zerbo, O., Boyce, T. G., Oster, M. E., Hanson, K. E., Donahue, J. G., Ross, P., Naleway, A., Nelson, J. C., Lewin, B., Glanz, J. M., Williams, J. T. B., Kharbanda, E. O., Katherine Yih, W., & Klein, N. P. (2022). Risk of myocarditis and pericarditis following BNT162b2 and mRNA-1273 COVID-19 vaccination. Vaccine, 40(35), 5153–5159. https://doi.org/10.1016/j.vaccine.2022.07.007
- Gee, J., Naleway, A., Shui, I., Baggs, J., Yin, R., Li, R., Kulldorff, M., Lewis, E., Fireman, B., Daley, M. F., Klein, N. P., & Weintraub, E. S. (2011). Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine, 29(46), 8279–8284. https://doi.org/10.1016/j.vaccine.2011.08.106
- Donahue, J. G., Kieke, B. A., Lewis, E. M., Weintraub, E. S., Hanson, K. E., McClure, D. L., Vickers, E. R., Gee, J., Daley, M. F., DeStefano, F., Hechter, R. C., Jackson, L. A., Klein, N. P., Naleway, A. L., Nelson, J. C., & Belongia, E. A. (2019). Near Real-Time Surveillance to Assess the Safety of the 9-Valent Human Papillomavirus Vaccine. Pediatrics, 144(6), e20191808. https://doi.org/10.1542/peds.2019-1808
- Sharon K. Greene, Martin Kulldorff, Edwin M. Lewis, Rong Li, Ruihua Yin, Eric S. Weintraub, Bruce H. Fireman, Tracy A. Lieu, James D. Nordin, Jason M. Glanz, Roger Baxter, Steven J. Jacobsen, Karen R. Broder, Grace M. Lee, Near Real-Time Surveillance for Influenza Vaccine Safety: Proof-of-Concept in the Vaccine Safety Datalink Project, American Journal of Epidemiology, Volume 171, Issue 2, 15 January 2010, Pages 177–188, https://doi.org/10.1093/aje/kwp345
- Lee, G. M., Greene, S. K., Weintraub, E. S., Baggs, J., Kulldorff, M., Fireman, B. H., Baxter, R., Jacobsen, S. J., Irving, S., Daley, M. F., Yin, R., Naleway, A., Nordin, J. D., Li, L., McCarthy, N., Vellozzi, C., Destefano, F., Lieu, T. A., & Vaccine Safety Datalink Project (2011). H1N1 and seasonal influenza vaccine safety in the vaccine safety datalink project. American journal of preventive medicine, 41(2), 121–128. https://doi.org/10.1016/j.amepre.2011.04.004
- Daley, M. F., Yih, W. K., Glanz, J. M., Hambidge, S. J., Narwaney, K. J., Yin, R., Li, L., Nelson, J. C., Nordin, J. D., Klein, N. P., Jacobsen, S. J., & Weintraub, E. (2014). Safety of diphtheria, tetanus, acellular pertussis and inactivated poliovirus (DtaP-IPV) vaccine. Vaccine, 32(25), 3019–3024. https://doi.org/10.1016/j.vaccine.2014.03.063.
- Klein, N. P., Fireman, B., Yih, W. K., Lewis, E., Kulldorff, M., Ray, P., Baxter, R., Hambidge, S., Nordin, J., Naleway, A., Belongia, E. A., Lieu, T., Baggs, J., Weintraub, E., & Vaccine Safety Datalink (2010). Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics, 126(1), e1–e8. https://doi.org/10.1542/peds.2010-0665
- Lieu, T. A., Kulldorff, M., Davis, R. L., Lewis, E. M., Weintraub, E., Yih, K., Yin, R., Brown, J. S., Platt, R., & Vaccine Safety Datalink Rapid Cycle Analysis Team (2007). Real-time vaccine safety surveillance for the early detection of adverse events. Medical care, 45(10 Supl 2), S89–S95. https://doi.org/10.1097/MLR.0b013e3180616c0a
- Nelson, J. C., Yu, O., Dominguez-Islas, C. P., Cook, A. J., Peterson, D., Greene, S. K., Yih, W. K., Daley, M. F., Jacobsen, S. J., Klein, N. P., Weintraub, E. S., Broder, K. R., & Jackson, L. A. (2013). Adapting group sequential methods to observational postlicensure vaccine safety surveillance: results of a pentavalent combination DTaP-IPV-Hib vaccine safety study. American journal of epidemiology, 177(2), 131–141. https://doi.org/10.1093/aje/kws317
- Weintraub, E. S., Baggs, J., Duffy, J., Vellozzi, C., Belongia, E. A., Irving, S., Klein, N. P., Glanz, J. M., Jacobsen, S. J., Naleway, A., Jackson, L. A., & DeStefano, F. (2014). Risk of intussusception after monovalent rotavirus vaccination. The New England journal of medicine, 370(6), 513–519. https://doi.org/10.1056/NEJMoa1311738
- Nelson, J. C., Ulloa-Pérez, E., Yu, O., Cook, A. J., Jackson, M. L., Belongia, E. A., Daley, M. F., Harpaz, R., Kharbanda, E. O., Klein, N. P., Naleway, A. L., Tseng, H. F., Weintraub, E. S., Duffy, J., Yih, W. K., & Jackson, L. A. (2023). Active Postlicensure Safety Surveillance for Recombinant Zoster Vaccine Using Electronic Health Record Data. American journal of epidemiology, 192(2), 205–216. https://doi.org/10.1093/aje/kwac170
- Yih, W. K., Nordin, J. D., Kulldorff, M., Lewis, E., Lieu, T. A., Shi, P., & Weintraub, E. S. (2009). An assessment of the safety of adolescent and adult tetanus-diphtheria-acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine, 27(32), 4257–4262. https://doi.org/10.1016/j.vaccine.2009.05.036
