At a glance
The False-Positive Investigation Toolkit provides mycobacteriology staff with resources to recognize potential false-positive results and to assist staff in false-positive investigations.

About this toolkit
False-positive Mycobacterium tuberculosis complex (MTBC) results can be difficult to identify, investigate, and resolve. False-positive results often have serious implications for patient isolation, patient therapy, contact investigations, and unnecessary laboratory testing. Errors leading to false- positive results can be classified as pre-analytic, analytic, or post-analytic. In any mycobacteriology laboratory, cross-contamination, mislabeling errors, specimen mix-ups, and use of nonsterile reagents can cause the inadvertent transfer of bacilli from one specimen or culture to another specimen or culture.
The False-Positive Investigation Toolkit provides mycobacteriology staff with updated resources to recognize potential false-positive results and to assist staff in false-positive investigations.
Download the False-Positive Investigation Toolkit
False-Positive Investigation Toolkit
Objectives
- Define false-positive results for MTBC.
- Identify best practices to prevent false-positive results for MTBC.
- Recognize possible scenarios for identifying potential false-positive results.
- Describe the actions necessary to investigate potential false-positive results.
- Summarize how the TB laboratory and the TB Control Program should collaborate to investigate false-positive results.
- Describe the importance of developing both laboratory and TB Control Program guidance, policies, and educational materials to be referenced by staff when necessary.
- Identify follow-up actions if a determination of false-positive result is made.
Who should use this toolkit
The toolkit's primary audience is mycobacteriology laboratory supervisors, technologists, and quality- assurance staff members. In addition, TB Control programs and healthcare providers may also find this information beneficial when investigating potential false-positive laboratory results.
What's included
The toolkit includes a document that can be reviewed and referred to and job aids, posters, and templates. The job aids and templates can be modified for local use and should not be sent to CDC for data collection.
Job Aids
- Pre-Analytic Checklist
- Prevention of False-Positive Results During Molecular Testing
- Situations or Scenarios That Might Initiate a Laboratory Investigation
- Laboratory Information to Monitor When Results Have the Potential to Be False-Positive
- Potential Areas to Examine if a False-Positive or Contamination is Suspected
Posters
- Do's and Don'ts to Prevent Cross-Contamination
- Do's and Don'ts to Prevent False-Positive Acid-Fast Bacilli (AFB) Smears
Templates
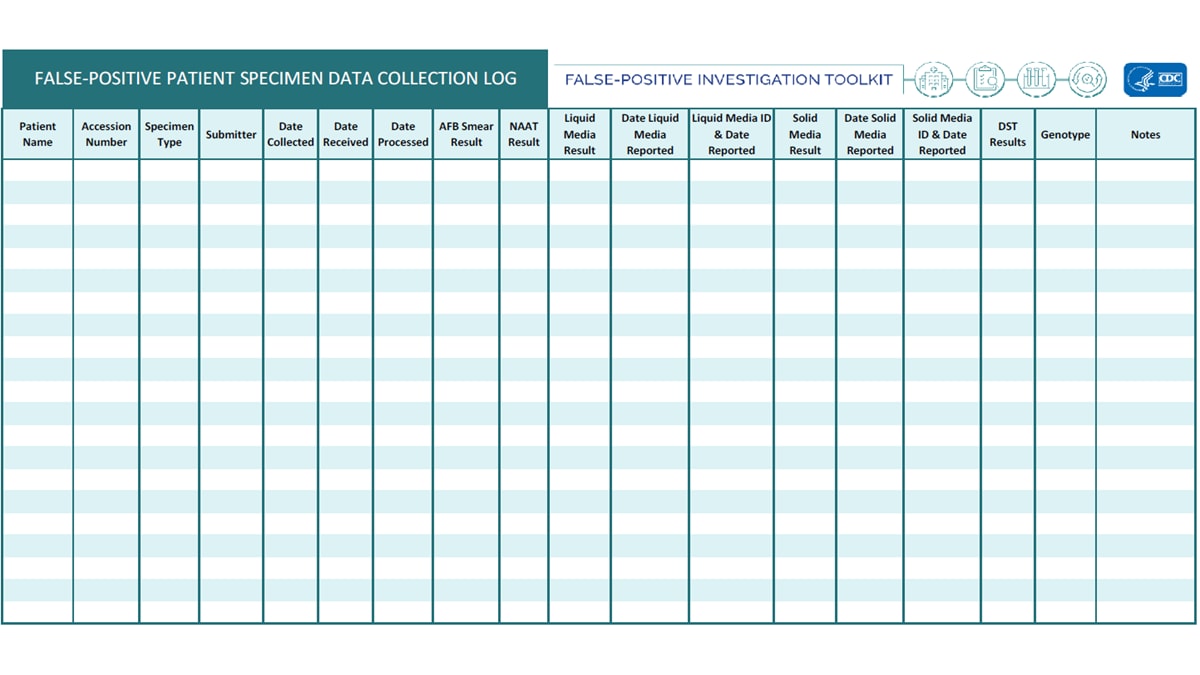
- False-Positive Patient Specimen Data Collection Log
- False-Positive Investigation Form
Case Study
An accompanying online interactive case study training module, Mycobacteriology False-Positive Case Studies, is available through the Association of Public Health Laboratories. The training module walks through different scenarios of a potential false-positive investigation.
Toolkit
False-Positive Investigation Toolkit: Pre-Analytic, Analytic, and Post Analytic Prevention Practices
False Positive Investigation Toolkit: Laboratory Monitoring
False-Positive Investigation Toolkit: Monitoring for False-Positive Results Outside the Laboratory
False-Positive Investigation Toolkit: Initial Communication of a Potential False-Positive
False-Positive Investigation Toolkit: Laboratory Areas for Investigations
False-Positive Investigation Toolkit: Actions Taken as a Result of an Investigation
Additional Information
AFB: Acid-fast bacilli
AFB culture: Acid-fast bacilli culture. The inoculation of a clinical specimen into culture media such as Becton Dickinson Mycobacteria Growth Indicator Tube (BD MGIT broth) or onto Middlebrook 7H11 or Lowenstein-Jensen (LJ) media slant with incubation at 36±1°C for up to six (6) to eight (8) weeks and detection or observation of growth or no growth during this incubation period.
AFB smear: Acid-fast bacilli smear. The microscopic examination of a Kinyoun, Ziehl-Neelsen, or fluorochrome stain of a clinical specimen.
BacTec MGIT™: Mycobacteria Growth Indicator Tube, Becton Dickinson and Co. A commercial, nonradiometric, broth-based mycobacterial culture system.
BSC: Biological safety cabinet
CAP: College of American Pathologists
Contamination: Inadvertent addition of target analytes to samples during sample collection, transportation, and analysis process
Cross-contamination: Act of spreading foreign or undesired material from one surface to another.
Epidemiologic links: Term used to identify whether individuals have a known connection through case interviews or contact investigations.
False-Positive: Error in which a test result improperly indicates presence of a condition, such as a disease.
Genotype: Genetic constitution of an individual organism used to distinguish different strains of bacteria.
Isolate: Culture growth of microorganisms isolated for testing.
LIMS: Laboratory Information Management System
MTBC: Mycobacterium tuberculosis complex
Nonconforming event (NCE): an unapproved departure (nonconformance) from a documented requirement or procedure (e.g., regulation, statute, policy, procedure, customer requirement)
NTM: Nontuberculous mycobacteria
PHL: Public health laboratory
PPE: Personal Protective Equipment. Equipment worn to minimize exposure to hazards that cause serious workplace injuries and illnesses.
Quality control (QC): Processes and products used to ensure that testing procedures are performed accurately by achieving expected results.
Specimen processing log: Log that is used in the laboratory to record when and how a specimen was processed, tested, and resulted.
Sputum: Mucous secretion from the lungs, bronchi, and trachea that is expectorated through the mouth.
TAT: Turnaround time
TB: Tuberculosis
The Clinical and Laboratory Standards Institute (CLSI) Laboratory Detection and Identification of Mycobacteria, 2nd ed. M48 Mycobacteriology Laboratory Guidance (2018) has been referenced throughout this toolkit1. Because of its broad use, this document should be consulted when further guidance is warranted. Additional references are cited as applicable.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of Health and Human Services.
Special thanks to U.S. state and local public health laboratories and TB Programs and the Association of Public Health Laboratories for content and critical review.
- CLSI, Laboratory Detection and Identification of Mycobacteria, 2nd ed., in CLSI guideline M48. 2018. Clinical and Laboratory Standards Institute: Wayne, PA.