Key points
This chapter provides detailed information regarding the post-licensure safety monitoring system VAERS (Vaccine Adverse Event Reporting System), established for collecting and analyzing adverse events following vaccination.
Public Health Importance
Vaccination is one of the ten great public health achievements of the 20th century.1 Vaccines have reduced the incidence of many vaccine-preventable diseases in the United States by more than 98% compared to the pre-vaccine era.23 This historic decrease in disease rates is shown in Table 1 below.
Vaccinations are usually administered to healthy persons and are often mandated by states as a condition for school attendance with certain exemptions allowed; therefore, they are held to a higher safety standard than other medical products.4 However, as with all medical products, no vaccine is completely safe or effective. Vaccines can cause minor adverse events (AEs), such as fever or local reactions at the injection site. Rarely, they can cause serious AEs such as anaphylaxis. Adverse events can also occur coincidentally after vaccines (i.e., they would have occurred without vaccination). Improving our understanding of vaccine safety is important to reduce the occurrence of vaccine AEs and maintain public confidence in vaccines. One way to enhance our understanding of vaccine safety is to improve surveillance for vaccine AEs. Robust vaccine safety monitoring may foster the discovery of AEs associated with vaccination and, thus, the development and use of safer vaccines, and recommendations to minimize the risk of AEs after vaccination (e.g., creating new recommendations, contraindications, and precautions).4
Table 1. Decline in vaccine-preventable disease annual morbidity in the United States from the baseline 20th century to 20201 23
| Disease | Baseline 20th century annual morbidity | 2020 reported cases11 | % Decrease |
|---|---|---|---|
| Smallpox | 48,1642 | 0 | 100 |
| Diphtheria | 175,8853 | 1 | >99 |
| Pertussis | 147,2714 | 6124 | >95 |
| Tetanus | 1,3145 | 17 | >98 |
| Poliomyelitis (paralytic) | 16,3166 | 0 | 100 |
| Measles | 503,2827 | 12 | >99 |
| Mumps | 152,2098 | 694 | >99 |
| Rubella | 47,7459 | 6 | >99 |
| Congenital rubella | 823 (estimated)10 | 0 | 100 |
| Haemophilus influenzae type b | 20,000 (estimated)11 | 136 (serotype b or unknown serotype, age <5 years) | >99 |
1 Disease data presented in the 2020 tables reflect the impacts of the COVID-19 pandemic, such as changes in exposure-related behavior, healthcare-seeking behavior, disease reporting, and public health investigations.
2 Average annual number of cases during 1900–1904.
3 Average annual number of reported cases during 1920–1922, 3 years before vaccine development.
4 Average annual number of reported cases during 1922–1925, 4 years before vaccine development.
5 Estimated cases based on reported deaths from 1922–1926 assuming a case-fatality rate of 90%.
6 Average annual number of reported cases during 1951–1954, 4 years before vaccine licensure.
7 Average annual number of reported cases during 1958–1962, 5 years before vaccine licensure.
8 Number of reported cases in 1968, the first year reporting began and the first year after vaccine licensure.
9 Average annual number of reported cases during 1966–1968, 3 years before vaccine licensure.
10 Estimated number of cases based on seroprevalence data in the population and on the risk that women infected during a childbearing year would have a fetus with congenital rubella syndrome.
11 Estimated number of cases from population-based surveillance studies before vaccine licensure in 1985.
Background
Vaccines, like other pharmaceutical products, undergo extensive testing and review for safety, immunogenicity, and efficacy in trials with animals and humans before they are licensed in the United States. Because these trials generally include a placebo control or comparison group, it is possible to ascertain which local or systemic reactions were due to the vaccine. However, prelicensure trials are relatively small and usually last no longer than a few years. In addition, they may be conducted in populations less demographically, racially, and ethnically diverse than those in which the vaccine is ultimately used. Persons with certain health conditions, such as pregnancy or immunosuppression, may be excluded from the trials. Prelicensure trials usually do not have the ability to detect rare AEs or an AE with delayed onset. The continuous monitoring of vaccine safety in the general population after licensure (known as post-licensure or post-marketing surveillance) is used to identify and evaluate risk for such AEs after vaccination.4
One post-licensure safety monitoring system is the Vaccine Adverse Event Reporting System (VAERS). VAERS, co-managed by the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA), was established in 1990 for collecting and analyzing reports of AEs following vaccination.5 Spontaneous or passive reporting systems for AEs similar to VAERS exist in many countries; some monitor vaccines separately from other drug products, but many are joint programs. These programs form the cornerstone of drug and vaccine safety monitoring efforts worldwide.
The Vaccine Life Cycle: Safety at Every Phase6
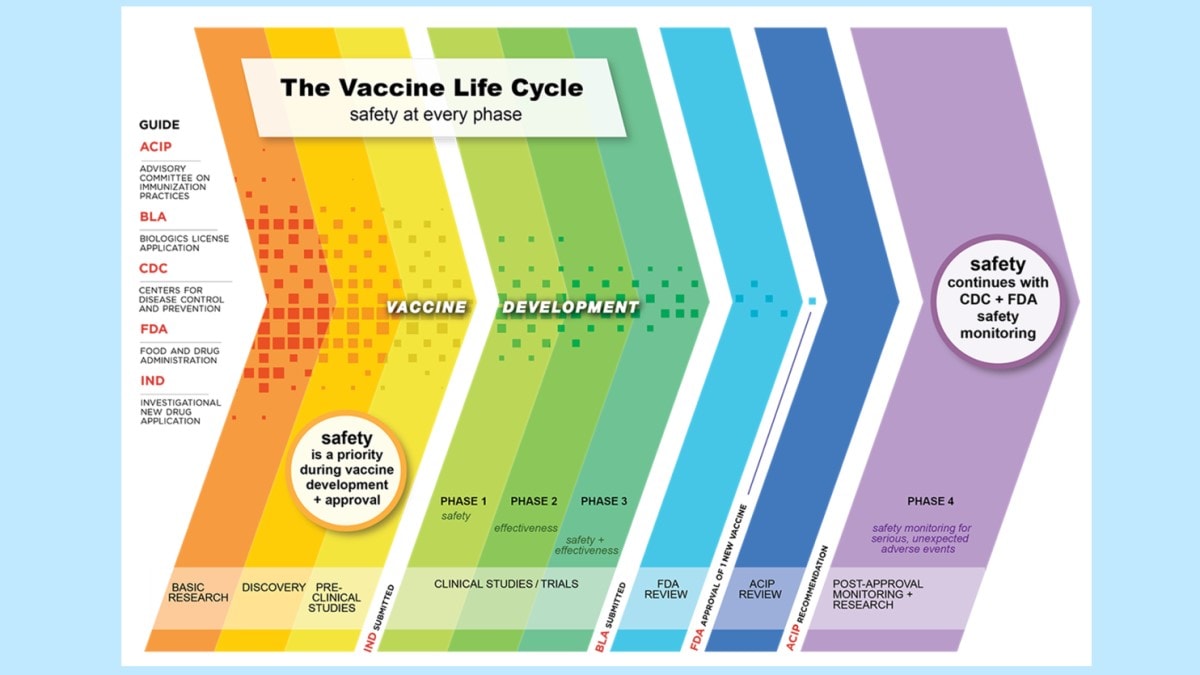
The National Childhood Vaccine Injury Act of 1986 (NCVIA)7 has the purpose of compensating people who may have been injured by vaccines and reducing threats to the stability of the immunization program (e.g., liability concerns, inadequate supply of vaccine, rising vaccine costs).8 The NCVIA requires that healthcare providers report specific AEs to VAERS (listed as a five-page document in the (VAERS) Table of Reportable Events Following Vaccination [5 pages]).9
While authorized under Emergency Use Authorization (EUA), certain AEs after COVID-19 vaccines were required to be reported to VAERS by healthcare providers as part of those authorizations and CDC COVID-19 Vaccination Provider Enrollment Agreements.10 These AEs should still be reported to VAERS. For a list of these AEs, please see VAERS—FAQs (hhs.gov)
Moreover, certain AEs after monkeypox vaccines are required to be reported to VAERS by healthcare providers as part of the FDA Emergency Use Authorization for Jynneos vaccine and the FDA Expanded Access Investigational New Drug (IND) mechanism for ACAM2000.11 For a list of AEs that should be reported to VAERS after monkeypox vaccines, please see VAERS—Report an Adverse Event (hhs.gov)
Objectives of VAERS
The objectives of VAERS are to:
- monitor for increases in known side effects, like arm soreness at the injection site;
- identify particular populations affected by health problems potentially related to vaccines;
- assess the safety of newly licensed or authorized vaccines;
- check for unexpected or unusual patterns of AE reports;
- identify rare AEs not detected during pre-licensure studies; and
- serve as a vaccine safety monitoring system during public health emergencies.
Scope of reports sought
Anyone can report an AE after vaccination to VAERS, including but not limited to healthcare providers, vaccine manufacturers, patients, and parents. Persons who are not healthcare providers are encouraged to consult with a healthcare provider to ensure that information is complete and accurate and that their provider is aware of the AE. Manufacturers are required to report to VAERS all AEs made known to them for any US-licensed vaccine. VAERS also accepts reports of vaccination errors.
As stated previously, the VAERS Table of Reportable Events Following Vaccination [5 pages] lists the adverse events after specific vaccines that are mandated for healthcare providers to report to VAERS. Additionally, certain adverse events that occur after COVID-19 vaccines and after monkeypox vaccines should be reported to VAERS (see VAERS—Report an Adverse Event (hhs.gov)). In addition, healthcare providers are encouraged to submit reports to VAERS for all clinically significant AEs occurring after vaccination, in all age groups, even if the causal relationship to vaccination is uncertain.
The VAERS form requests information about the AE, the vaccine(s) received, the timing of vaccination before the AE, demographic information about the recipient, concurrent medical illness and medications, prior medical history, and history of prior AEs to vaccinations. The VAERS form allows the reporter to describe the AE in a narrative format. The VAERS form was updated and the new form (VAERS-2.0) has been used since June 2017. The VAERS-2.0 form is available on the VAERS website.
The AE should be described on the VAERS form as clearly as possible, with accurate timing with respect to vaccination. The VAERS staff may request additional medical records or discharge summaries during follow-up for reports of a serious AE. Serious AEs, per federal law, are defined as death, a life-threatening AE, inpatient hospitalization or prolongation of existing hospitalization, persistent or significant incapacity or substantial disruption of the ability to conduct normal life functions, and congenital anomaly/birth defect.12
Reporting to VAERS
Reporting to VAERS online (i.e., web-based reporting) is strongly encouraged as it allows for quicker receipt and processing of information. The option to report using a downloadable, fillable PDF was made available in June 2017. If someone is unable to report online or via the writable pdf, they can contact VAERS by phone at 1-800-822-7967 or email at info@vaers.org for assistance.
A VAERS reporting form [4 pages], which can be copied for reporting purposes, is printed in Appendix 22. The form can also be requested by telephone at 800-822-7967. The Vaccine Information Statements (VIS) developed by CDC for all US-licensed vaccines and given to patients at the time of vaccination also contain information on how to report an AE to VAERS. Detailed instructions for completing the reporting form are provided below. Local health departments should follow the reporting instructions provided by their State Immunization Program.
Completion of VAERS form and submission of reports
Instructions for completing the VAERS form are on the VAERS website.
Note: Report AEs associated with vaccines on the VAERS form. Do not use the FDA's MEDWATCH forms to report vaccine AEs.
Do not report events associated with tuberculosis screening tests (Tine, PPD [purified protein derivative], or Mantoux), immune globulins, or other non-vaccine medical products to VAERS. Do not report events after monoclonal antibodies such as Beyfortus (nirsevimab) unless vaccines are given concurrently. Instead, these types of events should be reported to the MedWatch program online or by calling 800-FDA-1088 (800-332-1088).
Reporting responsibilities
Clinic staff at the local level should complete a VAERS report when an AE is suspected or occurs following immunization. As much of the requested information as possible should be included. Although reporting priority may be given to serious or unexpected events or unusual patterns of expected non serious events, all clinically significant AEs should be reported. Each report should be as complete and accurate as possible before it is sent to VAERS with specific attention to the following sections.
- Dates: All dates should make chronological sense. For example, the vaccine date cannot precede the birth date, and the report date cannot precede the vaccine date. All date fields should include the month, day, and year.
- Patient name: Verify that the patient's first and last names are correct. This check assists in identification of duplicate reports.
- Reporter information: Verify the reporter's name and contact information (such as address and phone number). VAERS staff send any reporter (other than a manufacturer) a letter or email (based on the reporter's preference) verifying receipt of the form and requesting any critical information that was missing from the VAERS report (if necessary).
- Critical boxes: Certain items on the VAERS form are crucial to the analysis of VAERS data and have been designated as critical boxes (data fields). Persons reporting will be asked to supply this information later if it is missing. Critical boxes are differentiated by a square around their respective item numbers on the form as follows and are highlighted on the pdf:
Table 2: VAERS 2.0 form critical box numbers
| Critical boxes | VAERS 2.0 Form box number |
|---|---|
| Date of birth | Box 2 |
| Sex | Box 3 |
| Date of vaccination (and time if known) | Box 4 |
| Date of onset of AE (and time, if known) | Box 5 |
| Age of patient at the time of vaccination | Box 6 |
| Narrative description of AE, symptoms, etc. | Box 18 |
All vaccines given on the date listed in box 4 of the VAERS-2.0 form, including:
|
Box 17 |
| Result or outcome of the AE, check all that apply (Indicates whether a report is regarded as serious or non-serious, and identifies the serious reports for follow-up)
Serious (serious status is based on the Code of Federal Regulations)
Non-serious
|
Box 21 |
- Timely reporting: Reporters are encouraged to send reports to VAERS as AEs occur, especially reports of any serious event. Programs are discouraged from sending batches of reports, which can delay reporting. Timely reporting is essential for rapid assessment of vaccine safety concerns and follow-up investigation. Public health jurisdictions are strongly encouraged to have providers send reports directly to VAERS without being first sent to the State Immunization Program staff. This practice increases the timeliness of the report.
Public health jurisdiction staff activities
The public health jurisdiction staff member designates a VAERS Coordinator or Vaccine Safety Coordinator with overall responsibility for VAERS-related activities including the following specific responsibilities.
- Serving as CDC's main point-of-contact for vaccine safety in the awardee's jurisdiction.
- Alerting CDC to vaccine safety concerns in awardee's jurisdiction and responding to vaccine safety emergencies.
- Reporting vaccine safety emergencies and events of concern requiring vaccine safety responses to the CDC Immunization Safety Office (404-498-0680) or the CDC Emergency Operations Center (770-488-7100).
- Collaborating with CDC and other partners (e.g., FDA, local health departments, healthcare facilities, providers) to respond to and investigate reports of serious adverse events in accordance with state health department policy.
- Identifying and responding to vaccine safety issues of concern in respective jurisdiction.
Evaluation of VAERS Data
VAERS contract site staff receive and process reports, which are entered into a database using a standard set of coding terms from the Medical Dictionary for Regulatory Affairs (MedDRA)13 to code the AEs. A report may include more than one AE. FDA and CDC medical officers and vaccine safety experts review reports of deaths and other serious events and conduct other analyses to address specific safety concerns and to evaluate trends in reporting. Although all serious reports are reviewed, it is primarily by analyzing all reports in aggregate that possible safety concerns (or "signals") between vaccines and AEs can be properly detected and assessed.5 When vaccine safety concerns are detected in VAERS they almost always require further assessment in other systems such as the Vaccine Safety Datalink (VSD) (see below).
With the use of the COVID-19 pandemic vaccines, the annual number of reports to VAERS increased significantly, from approximately 49,137 US reports in 2020, to approximately 752,660 US reports in 2021. Approximately 60,456 US reports of AEs following immunization were received by VAERS in the first six months of 2023 (CDC, unpublished data). All reports are accepted and entered without case-by-case determination of whether the AEs could have been caused by the vaccine in question. The increase in the number of VAERS reports received since the start of the COVID-19 pandemic reflects the huge increase in vaccination usage; from December of 2020 through May 11, 2023 (i.e., since the COVID-19 vaccines have been available for public use in the United States), over 676 million doses of COVID-19 vaccine doses were administered in the United States.14
Direct reporting to VAERS by healthcare providers or by State Immunization Program staff is strongly encouraged, as these reports usually arrive on a timelier basis than those submitted first to manufacturers. Manufacturers are not required to provide these reports to VAERS immediately upon receipt unless serious or unexpected events have occurred. As a result, evaluation of non-serious vaccine-associated events may be delayed.
Usefulness
The data from VAERS have been used by FDA, CDC, and the National Vaccine Injury Compensation Program at the Health Resources and Services Administration (HRSA), vaccine policy bodies, including the Advisory Committee on Immunization Practices (ACIP), and other stakeholders. Below are some examples of how VAERS data have contributed to public health, listed by some of the major objectives of VAERS.
- Detect new or rare AEs. Detection of greater than expected numbers of intussusception reports after introduction of the first rotavirus vaccine Rotashield is the classic example.15 Further investigation in other systems verified this association, and the Rotashield vaccine is no longer licensed in the United States.161718 A more recent example is when VAERS data contributed to important safety findings with the Janssen COVID-19 vaccine, including the rare but serious risk of thrombosis with thrombocytopenia syndrome19 and the increased risk of Guillain-Barre Syndrome (GBS).20 The Janssen COVID-19 vaccine is no longer available in the United States.
- Assess the safety of newly licensed vaccines. VAERS was an important tool used to assess the safety profile of COVID-19 vaccines authorized for emergency use during the COVID-19 pandemic.2122232425x
- Identify potential risk factors among populations affected by particular types of AEs. VAERS contributed data to support severe combined immunodeficiency syndrome (SCID) as a new contraindication for rotavirus vaccine.2627 Additionally, VAERS data was helpful in showing that there was an increased risk of myocarditis and pericarditis after mRNA COVID-19 vaccines, with the highest risk among males between the late teens and early 20's after receipt of dose 2 of the vaccine.28
- Rapidly respond to vaccine safety concerns or public health emergencies. VAERS provided the first national vaccine safety data during 2009–2010 H1N1 pandemic response29 and during the COVID-19 pandemic.21 VAERS has also identified a number of preventable vaccination errors that once brought to the attention of the public health community have been addressed in training materials and other prevention strategies.303132 Additionally, VAERS data were critical during the COVID-19 pandemic and were presented at several emergency meetings of the ACIP to provide data on safety of the new vaccines.33
VAERS data have also been used by the Health and Medicine division of the National Academies of Science, Engineering and Medicine [formerly called the Institute of Medicine (IOM)] Vaccine Safety Committee in an extensive assessment of the potential causal relationships between common childhood vaccines and AEs. IOM established an independent expert committee that conducted comprehensive reviews of 158 vaccine-AE pairs to study existing and emerging immunization safety concerns among eight different vaccines. In 2012, Adverse Effects of Vaccines: Evidence and Causality was published and included conclusions about causality for each vaccine-AE pair addressed. The IOM report summarized available epidemiologic evidence (including information obtained from VAERS) for causality between a vaccination and a hypothesized health effect, the biologic mechanisms relevant to the adverse event hypothesis, and the significance of the issue in a broader societal context. The entire report can be downloaded free of charge or purchased as a hard copy book. This reference may be useful to providers or public health officials who are called on to answer the public's questions on vaccine safety and the occurrence of AEs.34
More recently, (as previously stated), VAERS data has been shared in multiple publications to provide data on the safety and the signals of concern related to the pandemic COVID-19 vaccines.35
Reporting sensitivity
Like all passive surveillance systems, VAERS is subject to varying degrees of underreporting. The sensitivity of VAERS is affected by the likelihood that parents and/or vaccinees detect an AE; that parents and/or vaccinees bring the event to the attention of their healthcare provider(s); that parents and/or healthcare providers suspect an event is related to prior vaccination; that parents and/or healthcare providers are aware of VAERS; and that parents and/or healthcare providers report the event. The completeness of reporting of AEs associated with certain vaccines varies according to the severity of the event and the specificity of the clinical syndrome to the vaccine363738 Reporting can also be stimulated by media attention on specific AEs.39
Strengths of VAERS
VAERS data have several strengths, listed below:
- scale: VAERS is national in scope and, therefore, can be used during public health emergencies
- timeliness: reports received online are available to the CDC and FDA scientists within 24 hours after the report is received
- capacity to detect new, unusual, or rare AEs and to monitor known AEs observed during prelicensure trials
- ability to monitor newly licensed or newly authorized vaccines quickly
- accessibility: anyone can submit a report
Limitations of VAERS
The limitations of VAERS, which are common to many passive reporting systems, should be considered in interpreting VAERS data.
Lack of denominator data. Reporting rates can provide context for AEs observed after vaccination. Calculating reporting rates requires a denominator (i.e., a given number of AEs reported per one million vaccine doses). However, data on doses of vaccine administered are rarely available; data on doses of vaccine distributed can sometimes be used as a crude approximation of doses administered. When available, these data are often not age- or state- specific. Also, these data are proprietary and not available to the public. One exception is the seasonal influenza vaccine. Each year, data on the number of doses of influenza vaccine distributed are calculated by the CDC and made available to the public; these data are not product-specific by brand or manufacturer.40 Another exception was during the COVID-19 pandemic when the actual number of doses distributed, and doses administered by brand name were made available to the public.14
Quality of information. Since all reports, even incomplete ones, are accepted by VAERS, and because anyone may submit reports to VAERS, the accuracy and amount of information varies between reports.
Underreporting. Underreporting may occur for several reasons. These reasons include limitations in detecting an event, lack of recognition of a potential association between vaccine and event, or failure to submit a report. Underreporting can affect the ability of VAERS to detect very rare events. However, this may less of a concern for clinically severe events, which are more likely to be reported than non-serious events.36
Biased and stimulated reporting. Reports to VAERS may not be representative of all AEs that occur. Events that occur within a few days to weeks of vaccine administration are more likely to be submitted to VAERS than events with a longer onset interval. Additionally, media attention to particular types of medical outcomes can stimulate reporting.39
Confounding by drug and disease. Many reports to VAERS describe events that may have been caused by medications or underlying disease processes. Other reports to VAERS encompass clinical syndromes that are poorly defined, are not clearly understood, or represent diagnoses of exclusion (e.g., sudden infant death syndrome and myalgic encephalomyelitis/chronic fatigue syndrome).
Inability to determine causation. Because VAERS reports may lack unique laboratory findings or other information necessary to conclude, they are usually not helpful in assessing whether a vaccine caused a reported AE. Multiple vaccines are often administered at the same visit, making attributing causation to a single vaccine or antigen difficult. Additionally, there is a lack of an unvaccinated group for comparison in VAERS. Therefore, reports to VAERS are useful for generating hypotheses, but studies with vaccinated and unvaccinated subjects are necessary to confirm any hypotheses generated by VAERS observations.5
Enhancing Surveillance
Several activities can be undertaken to improve the quality of VAERS as a surveillance system.
Improving quality of information reported
VAERS forms (including the web-based reporting form) should be as complete and accurate as possible prior to submission. However, the VAERS report will be accepted regardless if information is missing. For death and serious outcomes after vaccination, the VAERS staff will attempt to obtain additional documentation (e.g., hospital discharge summaries, laboratory reports, death certificates, and autopsy reports). The VAERS staff contacts reporters — including health care providers and parents or vaccine recipients — to obtain missing information or to correct inaccurate information for all reports of deaths, serious AEs, and other selected clinically significant events. Mode of contact is usually by mail or email.
Promoting awareness
Current outreach and education efforts to promote VAERS include online print and web material, informational brochures in English and Spanish, and CDC vaccine safety publications.
VAERS contact information is included on all VISs, which are required to be provided at each vaccination visit to persons receiving a vaccine that is covered by the Vaccine Injury Compensation Program (VICP) ― (i.e., is listed on the Vaccine Injury Table). However, providing VISs to vaccine recipients for all vaccines, including those not covered by the VICP, is strongly encouraged.
Raw VAERS data files, without identifying information, are available to the public for download at the VAERS website. VAERS data are also available via search engines on the Wide-ranging Online Data for Epidemiologic Research (CDC WONDER site). Data on both sites are updated monthly and contain data from initial VAERS reports. However, data collected during follow-up is not included on the VAERS public data sites.
Despite its limitations, VAERS is useful in that it generates signals that trigger further investigations. VAERS can detect unusual increases in previously reported events. As noted earlier, the sentinel role of VAERS is particularly significant for new vaccines, as evidenced during the recent COVID-19 pandemic: many publications on COVID-19 vaccine safety contained VAERS data. These vaccines were new, authorized under EUA, and were administered to millions of people in a short period of time.41 Although manufacturers are now routinely asked to conduct or sponsor post-licensure studies designed to collect additional safety data for large numbers of vaccine recipients, the need for a national post-licensure surveillance system remains. Like prelicensure studies, post-licensure studies may not be large enough to detect novel or very rare AEs, may not include a varied study population, or may take several years to accumulate enough data to assess a rare occurrence.
The National Vaccine Injury Compensation Program and the Countermeasures Injury Compensation Program
The National Childhood Vaccine Injury Act of 1986 established the national VICP to provide compensation for AEs following immunization. VICP is a "no-fault" system to compensate individuals whose injuries may have been caused by covered vaccines. VICP is separate from VAERS and managed by a separate part of the federal government, the Health Resources and Services Administration (HRSA). Reporting an AE to VAERS does not result in filing a claim to the VICP. A compensation claim must be filed directly with VICP. Any individual of any age who received a covered vaccine and believes he or she was injured as a result can file a petition with VICP. The VICP's Vaccine Injury Table42 lists specific injuries or conditions and time frames following vaccination that may be compensated under the VICP. Suppose an injury and/or condition does not meet the requirements in the vaccine injury table. In that case a petitioner must prove through evidence, such as expert witness testimony, medical records, or medical opinion, that the vaccine caused the injury and/or condition.
The toll-free number for the VICP is 800-338-2382. Further information can be obtained by visiting their VICP website or writing to the National Vaccine Injury Compensation Program, Parklawn Building, 5600 Fishers Lane, 8N146B, Rockville, MD 20857.
The Countermeasures Injury Compensation Program (CICP) is a separate federal government "no-fault" program that compensates certain individuals seriously injured by covered countermeasures, such as COVID-19 vaccines, under declarations issued by the Secretary of the U.S. Department of Health and Human Services. Information on the CICP can be obtained by visiting the CICP website or calling 855-266-2427 (855-266-CICP).
Other Vaccine Safety Monitoring Activities
In addition to VAERS, several other systems exist to monitor the safety of vaccines. The systems maintained by the CDC are listed below.
The Vaccine Safety Datalink (VSD) is a collaborative effort between the CDC's Immunization Safety Office and several integrated healthcare systems to monitor immunization safety and address the gaps in scientific knowledge about AEs following immunization. The VSD links computerized vaccination and medical records for approximately 15.5 million persons (4.7% of the US population), enabling less frequent AEs to be evaluated. Denominator data and control groups are also readily available. The VSD thus provides a way of testing hypotheses related to vaccine safety. VSD has also implemented a system to conduct near real-time monitoring for specific AEs after vaccines in the VSD population.
The Clinical Immunization Safety Assessment (CISA) Project consists of seven academic centers with vaccine safety expertise working in partnership with CDC. It is designed to improve scientific understanding of vaccine safety issues at the individual patient level. The CISA Project's goals are to study mechanisms of vaccine AEs, study individual risk factors for AEs, serve as a resource to provide consultation for difficult vaccine safety issues, and to assist in developing vaccine safety guidance. The CISA Project is also engaged in clinical research.
V-safe is a surveillance system that CDC uses to monitor the safety of new or updated vaccines, such as the 2023–2024 COVID-19 and RSV vaccines. It provides quick, confidential health check-ins using text messages and web surveys to monitor for health problems after receipt of newly authorized or newly licensed vaccines.
In summary, ongoing post-licensure safety monitoring is necessary for all US-licensed vaccines. Well-established systems are in place to accomplish that monitoring and State Immunization Program staff play a key role in helping to ensure vaccines are safe.
Authors and Suggested Citation
Elaine R. Miller, RN, MPH; Tiffany Suragh, MPH; John R. Su, MD, PhD, MPH; Sandra Amouzou, MPH; Pedro Moro, MD, MPH
Suggested citation:
Given the variations in the timing for when chapter updates are made, a Manual edition number is no longer used. Therefore, it is recommended that the date at the top right of the web page be used in references/citations.
Content source:
National Center for Immunization and Respiratory Diseases
- CDC. Ten great public health achievements-United States, 1900-1999. MMWR Morb Mortal Wkly Rep 1999;48:241–3.
- CDC. Achievements in public health, 1900–1999. Impact of vaccines universally recommended for children— United States, 1990–1998. MMWR Morb Mortal Wkly Rep 1999;48:243–8.
- CDC. National Notifiable Diseases Surveillance System, 2020 Annual Tables of infectious disease data. Atlanta, GA. CDC Division of Health Informatics and Surveillance, 2023.
- Chen RT, Rastogi SC, Mullen JR, et al. The vaccine adverse event reporting system (VAERS). Vaccine 1994;12:542–50. doi: 10.1016/0264-410X(94)90315-8
- Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2015;33:4398–405. doi: 10.1016/j.vaccine.2015.07.035
- CDC. Vaccine safety: overview, history, and how the safety process works. 2020.
- National Childhood Vaccine Injury Act of 1986, at Section 2125 of the Public Health Service Act as codified at 42 U.S.C. Section 300aa.
- Brink EW, Hinman AR. The vaccine injury compensation act: the new law and you. In: Contemporary Pediatrics. Hoeson CP, Howe CJ, Finchere HV, editors. Institute of Medicne. 1989;6:(3)28–32,35–36,39–42.
- HRSA. VAERS table of reportable events following vaccination. 2017.
- U.S. Department of Health & Human Services, Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases. CDC COVID-19 vaccination program provider requirements and support. 2022.
- Key facts about vaccines to prevent monkeypox disease. 2022.
- Code of Federal Regulations Title 21, Part 312, Subpart B.
- Medical Dictionary for Regulatory Activities (MedDRA).
- COVID data tracker. 2022.
- Intussusception among recipients of rotavirus vaccine—United States, 1998–1999. MMWR Morb Mortal Wkly Rep 1999;48(27):577–81.
- Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001;344(8):564–72. doi: 10.1056/NEJM200102223440804
- Zanardi LR, Haber P, Mootrey GT, Niu MT, Wharton M. Intussusception among recipients of rotavirus vaccine: reports to the Vaccine Adverse Event Reporting System. Pediatrics 2001;107(6):E97.
- Kramarz P, France EK, DeStefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J 2001;20(4):410–6.
- See I, Lale A, Marquez P, Streiff MB, Wheeler AP, Tepper NK, et al. Case series of thrombosis with thrombocytopenia syndrome after COVID-19 vaccination—United States, December 2020 to August 2021. Ann Intern Med. 2022;175:513–22. doi: 10.7326/M21-4502.
- Abara WE, Gee J, Marquez P, Woo J, Myers TR, DeSantis A, et al. Reports of Guillain-Barré Syndrome after COVID-19 vaccination in the United States. JAMA Netw Open 2023;6:e2253845. doi: 10.1001/jamanetworkopen.2022.53845.
- Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring —United States, December 14, 2020–January 13, 2021. MMWR Morb Mortal Wkly Rep 2021;70:283–8. doi: 10.15585/mmwr.mm7008e3.
- Moro PL, Panagiotakopoulos L, Oduyebo T, Olson CK, Myers T. Monitoring the safety of COVID-19 vaccines in pregnancy in the US. Hum Vaccin Immunother 2021;17:4705–13. doi: 10.1080/21645515.2021.1984132.
- Moro PL, Zhang B, Ennulat C, Harris M, McVey R, Woody G, et al. Safety of co-administration of mRNA COVID-19 and seasonal inactivated influenza vaccines in the vaccine adverse event reporting system (VAERS) during July 1, 2021-June 30, 2022. Vaccine 2023;41:1859–63. doi: 10.1016/j.vaccine.2022.12.069.
- Shimabukuro T, Nair N. Allergic reactions Including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. Jama. 2021;325:780–1. doi: 10.1001/jama.2021.0600.
- Woo EJ, Gee J, Marquez P, Baggs J, Abara WE, McNeil MM, et al. Post-authorization safety surveillance of Ad.26.COV2.S vaccine: Reports to the Vaccine Adverse Event Reporting System and v-safe, February 2021–February 2022. 2023;41:4422–30. doi: 10.1016/j.vaccine.2023.06.023.
- Bakare N, Menschik D, Tiernan R, Hua W, Martin D. Severe combined immunodeficiency (SCID) and rotavirus vaccination: reports to the Vaccine Adverse Events Reporting System (VAERS). Vaccine 2010; 28(40):6609–12. doi: 10.1016/j.vaccine.2010.07.039
- Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep 2010;59(22):687–8.
- Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US From December 2020 to August 2021. Jama 2022;327:331–40. doi: 10.1001/jama.2021.24110
- Safety of influenza A (H1N1) 2009 monovalent vaccines—United States, Oct. 1–Nov.24, 2009. MMWR Morb Mortal Wkly Rep 2009; 58(48):1351–6.
- Hibbs BF, Moro PL, Lewis P, Miller ER, Shimabukuro TT. Vaccination errors reported to the Vaccine Adverse Event Reporting System, (VAERS) United States, 2000—2013. Vaccine 2015;33(28):3171–8. doi: 10.1016/j.vaccine.2015.05.006
- Su JR, Miller ER, Duffy J, Baer BM, Cano MV. Notes from the field: administration error involving a meningococcal conjugate vaccine—United States, March 1, 2010–September 22, 2015. MMWR Morb Mortal Wkly Rep 2016;65(6):161–2.
- Haber P, Schembri CP, Lewis P, et al. Notes from the field: reports of expired live attenuated influenza vaccine being administered—United States, 2007–2014. MMWR Morb Mortal Wkly Rep 2014;63(35):773.
- Advisory Committee on Immunization Practices. ACIP Meeting Information.
- Institute of Medicine. Adverse effects of vaccines: evidence and causality. Stratton K, Ford A, Rusch E, Clayton EW, editors. Washington, DC: National Academies Press; 2011.
- Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Vaccine Adverse Event Reporting System (VAERS) Publications. 2023.
- Rosenthal S, Chen R. The reporting sensitivities of two passive surveillance systems for vaccine adverse events. Am J Public Health 1995;85(12):1706–9. doi: 10.2105/AJPH.85.12.1706
- Verstraeten T, Baughman AL, Cadwell B, Zanardi L, Haber P, Chen RT, Vaccine Adverse Event Reporting System Team. Enhancing vaccine safety surveillance: a capture-recapture analysis of intussusception after rotavirus vaccination. Am J Epidemiol 2001;154(11):1006–12. doi: 10.1093/aje/154.11.1006
- Miller ER, McNeil MM, Moro PL, Duffy J, Su JR. The reporting sensitivity of the Vaccine Adverse Event Reporting System (VAERS) for anaphylaxis and for Guillain-Barre syndrome. Vaccine 2020;38:7458–63. doi: 10.1016/j.vaccine.2020.09.072.
- Eberth JM, Kline KN, Moskowitz DA, Montealegre JR, Scheurer ME. The role of media and the Internet on vaccine adverse event reporting: a case study of human papillomavirus vaccination. J Adolesc Health 2014;54(3):289–95. doi: 10.1016/j.jadohealth.2013.09.005
- Influenza Vaccine Doses Distributed, United States. 2023.
- Rosenblum HG, Gee J, Liu R, Marquez PL, Zhang B, Strid P, et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: an observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect Dis 2022;22:802–12. doi: 10.1016/S1473-3099(22)00054-8.
- Vaccine Injury Table. 2022.
