At a glance
- To better understand health issues and needs among people with spina bifida, CDC collects data through the National Spina Bifida Patient Registry.
- CDC also leads research efforts to understand bladder and kidney issues for people with spina bifida.
- CDC tracks the number of people affected by spina bifida and health issues across their lifespan.

National Spina Bifida Patient Registry
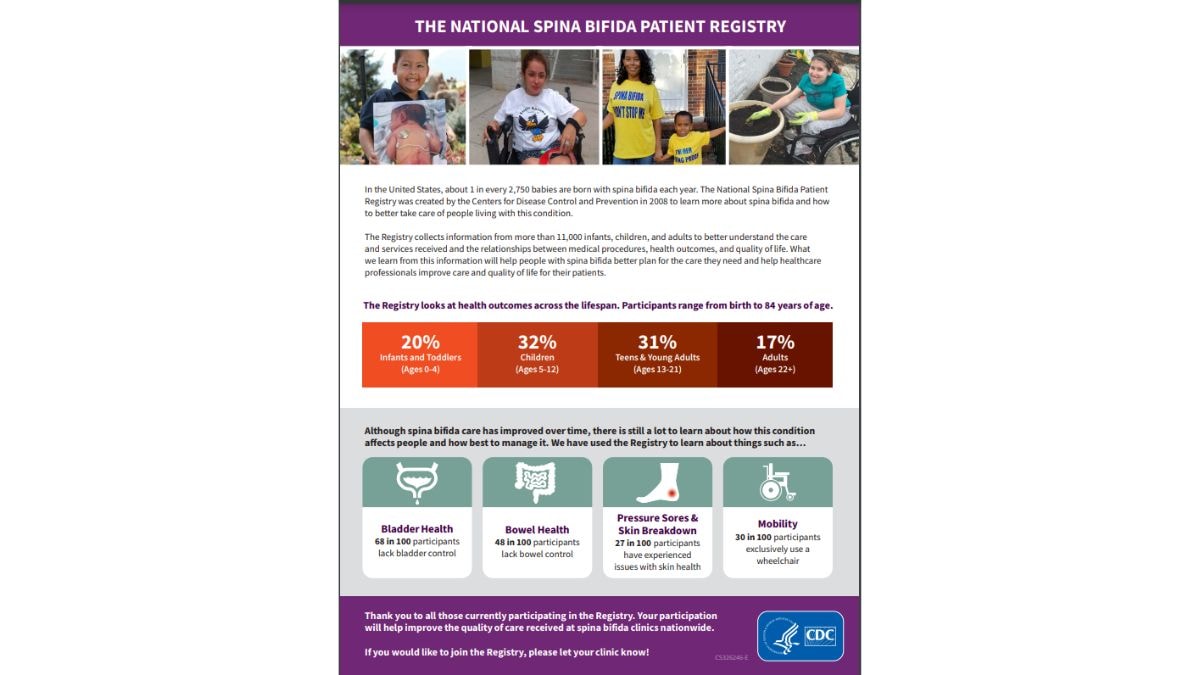
CDC funds and manages the National Spina Bifida Patient Registry (NSBPR), which was established in 2008. Data gathered in the NSBPR come from children and adults who attend participating spina bifida clinics in the United States. These data document the care patients receive and the outcomes of that care.
The purpose of the NSBPR is to collect clinical data to evaluate existing medical services for spina bifida patients. The NSBPR also provides the framework to improve the quality of care received at spina bifida clinics nationwide.
The NSBPR collects data on patient demographics, treatment, and outcomes (bowel and bladder continence, mobility, skin problems). Findings from the NSBPR can be used to:
- Develop standards of care and treatment for patients with spina bifida
- Revise standards of care and treatment when necessary
- Share evidence-based information between clinicians across the country
- Implement benchmarks to improve care in spina bifida clinics
- Identify centers that provide the most beneficial care to patients
More information
Urologic protocol for young children (UMPIRE)
Many babies born with spina bifida have healthy kidneys at birth.1 However, they may develop urinary tract infections or kidney failure at a younger age than those without spina bifida.2 To monitor how well the bladder and kidneys are working in infants and children with spina bifida, CDC and other experts developed the Urologic Management to Preserve Initial Renal Function (UMPIRE) protocol. Started in 2014, researchers plan to follow infants until they reach 10 years of age.
The regular monitoring and testing developed through the UMPIRE project helps identify kidney disease earlier. Early detection may keep kidney disease from getting worse.
Research
Aims for UMPIRE data include:
- Identify whether infants with myelomeningocele routinely need preventive antibiotics to avoid urinary tract infections
- Standardize how clinicians administer and interpret common bladder and kidney tests
- Determine the characteristics to help identify which children born with myelomeningocele are likely to have kidney problems
- Evaluate differences in cost of care and health outcomes between patients included in UMPIRE and patients not included
Birth defects tracking and research
Tracking Spina Bifida Across the Lifespan (SB STAR)
Traditionally, tracking birth defects like spina bifida involves identifying and documenting affected babies at birth. However, these systems often do not continue monitoring these infants into childhood or adulthood. Thanks to advances in medical care, people with spina bifida are now living longer. Despite this, we know little about how many people are living with spina bifida in the United States or what health issues they experience across their lifespan.
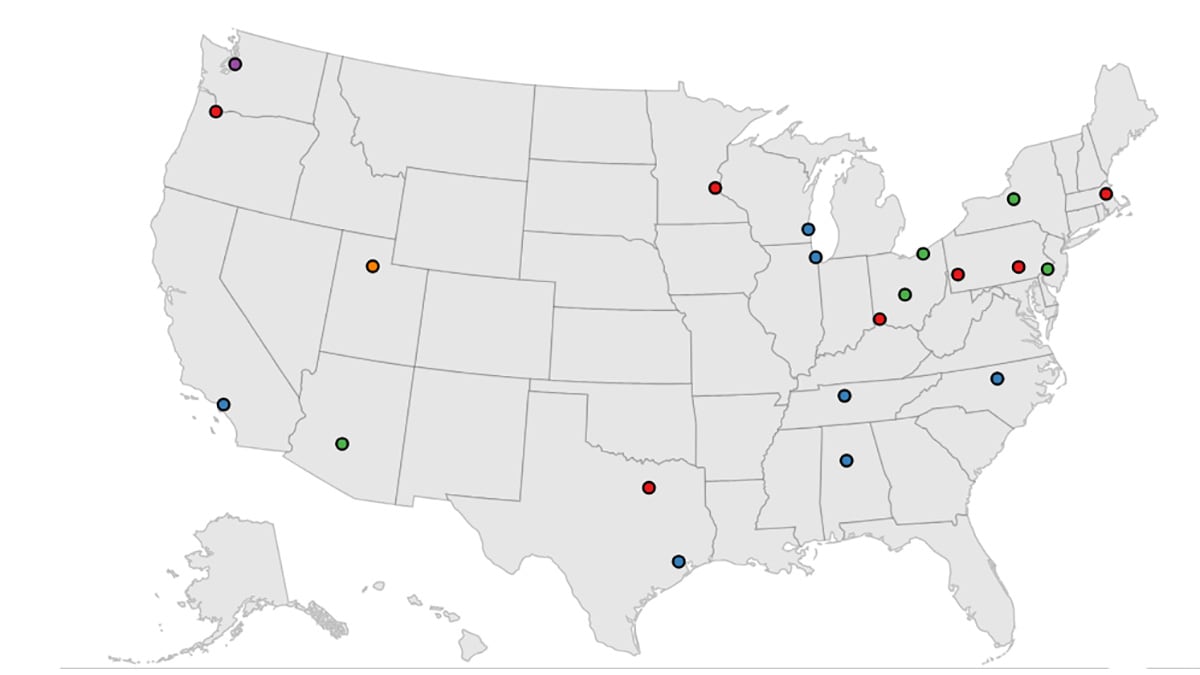
In 2024, CDC funded the Spina Bifida Surveillance across Time and Regions (SB STAR) project. Four recipients were selected for funding: the University of Arizona, University of Minnesota, New York State Department of Health, and University of Utah. The sites will collect health data on persons with spina bifida of all ages who live in these areas, regardless of whether they receive care at spina bifida specialty clinics. The sites will gather health information from birth defects registries, medical clinics, hospitals, birth and death certificates. The goal of SB STAR is to enhance the quality of life for people living with spina bifida.
Spina Bifida Collaborative Care Networks (SBCCN)
The goal of the SBCCN is to improve the health of people living with spina bifida across their lifespan by identifying needs of people with spina bifida, connecting them with high quality health care providers, and identifying research gaps and priorities. This work is executed in partnership with the Spina Bifida Association (SBA).
- Tanaka ST, Paramsothy P, Thibadeau J, Wiener JS, Joseph DB, Cheng EY, et al.Baseline urinary imaging in infants enrolled in Urologic Management to Preserve Initial Renal Function (UMPIRE) protocol for children with spina bifida.J Urol. 2019 Feb 5.
- Ouyang L, Bolen J, Valdez R, Joseph D, Baum MA, Thibadeau J.Characteristics and survival of patients with end stage renal disease and spina bifida in the United States renal data system.J Urol. 2015 Feb;193(2):558-64.