Key points
- The smallpox vaccine is generally safe and effective, but severe adverse reactions are common in some people.
- Vaccinia immune globulin intravenous (VIGIV) is recommended as the first line of therapy for treatment of adverse reactions resulting from continued vaccinia virus replication after vaccination using ACAM2000® or APSV.
- Antivirals may be considered as a secondary treatment after consultation with CDC.
Overview
The smallpox vaccine is generally safe and effective, but some people do experience side effects and adverse reactions. Severe adverse reactions are more common in people who are being vaccinated for the first time and among young children (<5 years of age).
CDC has published criteria to use for surveillance case definition and classification for smallpox vaccine (vaccinia) adverse reactions. CDC also provides consultation for clinicians to help diagnose and manage patients with suspected vaccinia virus vaccine adverse reactions. Clinicians may reach CDC medical staff by calling the Emergency Operations Center at 770-488-7100.
Localized reactions
Superinfection of the vaccination site or regional lymph nodes
Vaccination progression and normal local reactions are difficult to distinguish from a superinfection of the vaccination site or regional lymph nodes. Secondary infections of the vaccination site are uncommon and are typically mild to moderate in clinical severity. Children and individuals who frequently manipulate and contaminate the vaccination site are at greatest risk.
Robust take
A robust take is a vaccinial cellulitis and is defined as >3 inches of redness with swelling, pain, and warmth at the vaccination site. The symptoms peak 6 to 12 days after vaccination and regress within the following 24 to 72 hours.
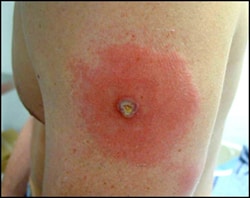
Unintentional transfer of vaccinia virus
Inadvertent autoinoculation
Inadvertent autoinoculation is the unintentional transfer of vaccinia virus from the vaccination site to another place on the vaccinee's body. Vaccinees can transfer vaccinia to their hands or fomites. The most common sites are the eye and surrounding orbit (ocular vaccinia), followed by the face, nose, mouth, lips, genitalia, and anus.
Contact transmission
A vaccinee can also spread vaccinia virus from the vaccination site (or other lesions distant from the vaccination site) to close contacts through direct contact or through other vectors such as clothing, bedding, or bandages contaminated by vaccinia virus. Viral shedding can occur until the scab detaches from the vaccination site (or any distant lesions), revealing healthy skin underneath. Infection through contact transmission can result in the same adverse events observed after smallpox vaccination.
Ocular vaccinia
There are different forms of ocular vaccinia: blepharitis (inflammation of the eyelid), conjunctivitis, keratitis (inflammation of the cornea, including epithelial and stromal forms), iritis, or combinations thereof. Infections can be clinically mild to severe and can lead to vision loss.
Diffuse dermatologic complications
There are two groups of diffuse dermatological complications: those thought to be free of vaccinia virus (e.g., erythema multiforme minor, Stevens-Johnson syndrome, and other nonspecific post-vaccination rashes), and those thought to be caused by replicating vaccinia virus at the site of the skin lesions. This section covers the second group.
Generalized vaccinia
Generalized vaccinia is a disseminated vesicular or pustular rash and is usually benign and self-limited among immunocompetent hosts. Modern case definitions call for the presence of lesions removed from the initial vaccination site and laboratory evidence supporting the diagnosis of vaccinia virus infection and/or excluding other causes of papular or vesicular eruptions. First-time vaccinees are at higher risk for generalized vaccinia than revaccinees. Generalized vaccinia is often more severe among persons with underlying immunodeficiency who might have been inadvertently vaccinated.
Eczema vaccinatum
Eczema vaccinatum is a localized or systemic spread of vaccinia virus. It occurs most often in vaccine recipients who have a history of atopic dermatitis. The rash is often accompanied by fever and lymphadenopathy, and affected persons are frequently systemically ill. Eczema vaccinatum tends to be most severe among first-time vaccinees, unvaccinated close contacts of vaccinees, and young children. It can be fatal.
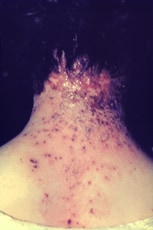
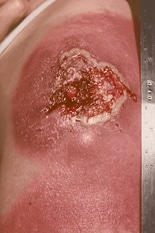
Progressive vaccinia
Progressive vaccinia is rare, severe, and often fatal. It occurs when a vaccination site fails to heal and vaccinia virus replication persists. The skin surrounding the vaccination site becomes infected with vaccinia virus, and secondary metastatic vaccinia lesions can occur. Lesions can appear necrotic, fungated, piled-up, or well demarcated. Concomitant bacterial superinfection also can occur. Progressive vaccinia typically occurs in persons with an underlying humoral or cellular immune deficit.
Rare adverse reactions
Fetal vaccinia
Rarely, smallpox vaccination of a pregnant woman can result in fetal vaccinia. Transmission to the fetus can occur any time during pregnancy. Miscarriage, stillbirth, or live birth (usually premature, followed by death) or birth of a surviving but pox-scarred infant can occur after the mother's exposure to vaccinia.
Postvaccinial central nervous system disease
Postvaccinial central nervous system disease such as postvaccinial encephalitis or encephalomyelitis may occur. It is most common among infants aged less than 12 months. Clinical symptoms reflect cerebral or cerebellar dysfunction with headache, fever, vomiting, altered mental status, lethargy, seizures, and coma. No clinical criteria, radiologic findings, or laboratory tests that are diagnostic for these adverse reactions exist
Cardiac adverse events
Myo/pericarditis
Reports of inflammatory disease of the myocardium, pericardium, or both after smallpox vaccination suggest an association between smallpox vaccination with the New York City Board of Health vaccinia strain and myo/pericarditis. Clinical presentation may include chest pain, dyspnea, and palpitations that range from subtle to severe. The case definition found in the Surveillance Guidelines for Smallpox Vaccine (vaccinia) Adverse Reactions provides more information about the spectrum of abnormalities found in inflammatory heart disease.
Dilated cardiomyopathy and cardiac ischemia
Dilated cardiomyopathy and cardiac ischemia have been noted in temporal association to smallpox vaccination, but have not been demonstrated to be linked etiologically. Dilated cardiomyopathy is a known sequelae of viral myocarditis and can present weeks to months after acute infection. The causal relation between smallpox vaccination and dilated cardiomyopathy is unclear, but is biologically plausible
Vaccine Adverse Event Reporting
Medical management of adverse reactions to replication-competent vaccinia virus vaccination
Vaccinia immune globulin intravenous (VIGIV) is recommended as the first line of therapy for treatment of adverse reactions resulting from continued vaccinia virus replication after vaccination using ACAM2000® or APSV. Antivirals may be considered as a secondary treatment after consultation with CDC. Clinicians who need assistance with the diagnosis and management of patients with suspected complications of vaccinia virus vaccination should consult with their state/local public health department. CDC will also provide consultation if requested. Contact CDC medical staff by calling the CDC Emergency Operations Center at 770-488-7100.
Vaccinia immune globulin intravenous (human) (VIGIV)
VIGIV [PDF – 18 pages] has been used safely and effectively in smallpox vaccinated individuals to treat adverse reactions that are secondary to continued vaccinia virus replication after vaccination. VIGIV is licensed by FDA for the treatment of following complications resulting from smallpox vaccination:
- Eczema vaccinatum
- Progressive vaccinia
- Severe cases of generalized vaccinia
- Vaccinia infections in individuals who have skin conditions such as burns, impetigo, varicella-zoster, or poison ivy; or in individuals who have eczematous skin lesions because of either the activity or extensiveness of such lesions
- Aberrant infections induced by vaccinia virus that include its accidental implantation in eyes (except in cases of isolated keratitis), mouth, or other areas where vaccinia infection would constitute a special hazard
VIGIV is not indicated for the treatment of isolated vaccinia keratitis or postvaccinial encephalitis. It is not indicated for the treatment of smallpox disease. VIGIV is not commercially available but can be made available through the Strategic National Stockpile (SNS) for the treatment of smallpox vaccine complications in patients with serious clinical manifestations.
Antivirals
If treatment with VIGIV alone is inadequate or if VIGIV is not readily available, the following three antivirals may be considered, based on clinical determination for antiviral treatment. No antivirals are currently approved by the FDA for treatment of complications which might arise from vaccinia vaccination, but some may be used under expanded access investigational new drug (IND) protocols.
- Tecovirimat (also referred to as ST-246 or its brand name Tpoxx) has demonstrated in vitro activity against various orthopoxviruses (e.g., variola, vaccinia, and monkeypox viruses) and shown effectiveness in animal challenge studies using related orthopoxviruses. This antiviral is FDA-approved for the treatment of variola virus infections (smallpox) and could be used under an expanded access investigational new drug (IND) protocol for the treatment of adverse reactions secondary to continued vaccinia virus replication after smallpox vaccination. Tecovirimat has been used in a small number of individuals to date for the treatment of severe adverse events resulting from vaccinia virus vaccination, and effectiveness data in humans is limited. Tecovirimat is known to have fewer side effects than cidofovir.
- Brincidofovir (TEMBEXA) has demonstrated in vitro activity against various orthopoxviruses (e.g., variola, vaccinia, and monkeypox viruses) and shown effectiveness in animal challenge studies using related orthopoxviruses. This antiviral is FDA-approved for the treatment of variola virus infections (smallpox). Brincidofovir may have fewer adverse events than cidofovir (which is FDA-approved for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome). Patients being treated with cidofovir have experienced serious renal toxicity and other adverse events that have not been observed in patients being treated with brincidofovir.
- Cidofovir has shown effectiveness in animal and in vitro studies; however, there are limited data on the effectiveness in the treatment of vaccinia-related complications in humans. There are also limited data on the effectiveness in treatment of smallpox disease. Cidofovir is not FDA-approved for the treatment of any orthopoxvirus infections, including smallpox or vaccinia-related complications, but is commercially available. Clinicians could choose to use cidofovir to treat patients with orthopoxvirus infection based on individual risk-benefit assessment under practice of medicine.
Currently, VIGIV, tecovirimat, and brincidofovir are stockpiled by the SNS and would be made available under the appropriate regulatory mechanism.
| Drug | FDA approved for adverse reactions to smallpox (vaccinia) vaccine? | Available in Strategic National Stockpile? | Regulatory mechanism for treatment of adverse reactions to smallpox (vaccinia) vaccine? |
| VIGIV | Yes | Yes | FDA-approved use |
| Tecovirimat | No | Yes | IND protocol |
| Cidofovir | No | No (commercially available) | N/A (practice of medicine) |
| Brincidofovir | No | Yes | Single-patient emergency IND |
Obtaining VIGIV and Antivirals
Clinicians who need clinical consultation for patients experiencing a severe or unexpected adverse reaction following vaccinia virus vaccination, or who would like to request release of VIGIV or antivirals, should contact their state/local health department or the CDC Emergency Operations Center at 770-488-7100. If it is determined that treatment of vaccinia virus vaccine adverse reactions requires VIGIV or antivirals, the CDC Smallpox Vaccine Adverse Events Clinical Consultation Team will coordinate shipment with the Strategic National Stockpile.
Health care providers at military medical facilities (or civilian providers treating a U.S. Department of Defense healthcare beneficiary) should call the Defense Health Agency's 24/7 Immunization Healthcare Support Center at 877-GETVACC (877-438-8222) and select option #1.
Resources
- Smallpox Vaccination and Adverse Reactions: Guidance for Clinicians, 2003 Source: MMWR 2003, 52(RR04);1-28.
- Smallpox Vaccination and Adverse Reactions: Guidance for Clinicians, 2003 Source: MMWR 2003, 52(RR04);1-28. (Note: This guidance has references to Dryvax®, which is no longer available. However, guidance is relevant for all U.S. vaccine formulations that contain the New York City Board of Health vaccinia strain which includes ACAM2000® and APSV.)
- Surveillance Guidelines for Smallpox Vaccine (vaccinia) Adverse Reactions, 2006 Source: MMWR 2006, 55(RR01);1-16.
- Smallpox Vaccination and Adverse Reactions: Guidance for Clinicians, 2003 Source: MMWR 2003, 52(RR04);1-28.
- TPOXX® Printed Labeling [PDF- 21 pages]
- TEMBEXA® Printed Labeling [PDF- 21 pages]
