Key points
- High-risk specimens/cases require consultation with CDC prior to testing.
- Low-or moderate-risk specimens/cases should be worked-up for common causes of febrile exanthema.
- Due to differences in the thermocycling conditions used for the Non-variola Orthopoxvirus and Orthopoxvirus PCR assays, they cannot be performed simultaneously on the same instrument.
Overview
This protocol has been developed to guide the sequence and type of laboratory testing to be undertaken in situations involving specimens from patients with acute, generalized vesicular or pustular rash illness, or suspected smallpox vaccine (vaccinia virus) adverse event. This protocol may also be followed to test environmental specimens which may contain an Orthopoxvirus. The protocol has been designed for use alongside the Centers for Disease Control and Prevention's clinical assessment tool, Evaluating Patients for Smallpox: Acute, Generalized Vesicular or Pustular Rash Illness Protocol. The laboratory testing protocol is designed to address testing needs in a pre-event setting, when no poxvirus emergency has been detected or declared. In the event of a smallpox (variola virus) outbreak, critical updates will be announced by the Laboratory Response Network (LRN).
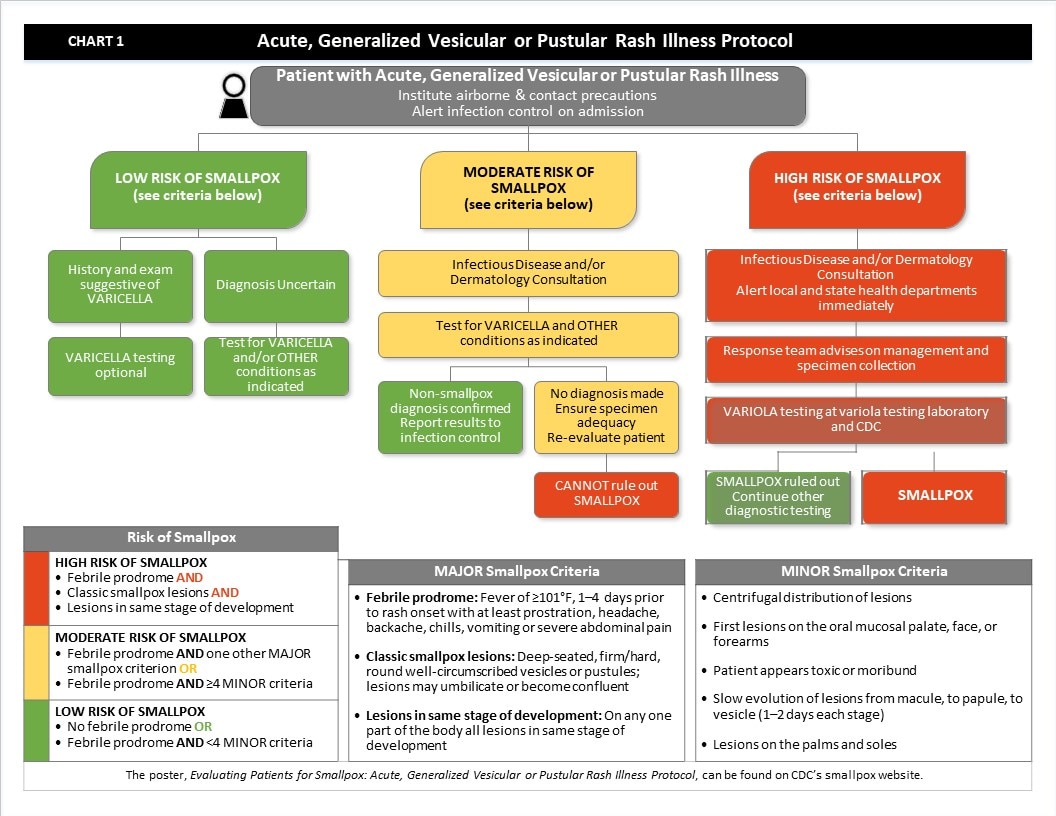
Chart 1 (below) lists the major and minor criteria of smallpox used to categorize a patient's risk of smallpox – high, moderate, or low.
The sequence and type of laboratory tests performed on specimens from patients with acute, generalized vesicular or pustular rash illness will depend on the outcome of the risk assessment using the clinical assessment tool, Evaluating Patients for Smallpox: Acute, Generalized Vesicular or Pustular Rash Illness Protocol (see Chart 1 for abstraction of the protocol).
When examining specimens from low- or moderate-risk cases it may be possible, though unlikely, that a positive result may be obtained using the Orthopoxvirus PCR assay and a negative result with Non-variola Orthopoxvirus PCR assay. This could signal a laboratory error, the presence of a previously uncharacterized Orthopoxvirus in the specimen, or that the patient has modified smallpox. Consultation with CDC should occur before running Variola virus specific PCR assays on specimens not initially determined to be high-risk.
The testing protocols are supported at the LRN reference laboratories. Charts to assist with the sequence of laboratory testing, smallpox vaccine adverse events, and environmental specimens are available through the LRN as needed.
Details on the performance and interpretation of each assay are specified in each LRN procedure. Communication between laboratories and local or state epidemiologists is encouraged prior to laboratory testing, especially if high-risk specimens are involved. Refer to LRN policy regarding notification of test results.
Chart 1
-
-
- When a patient presents with an acute, generalized vesicular or pustular rash illness, institute airborne and contact precautions. Alert infection control on admission.
- Determine the patient’s risk of smallpox using the MAJOR and MINOR criteria.
- There are three MAJOR criteria:
- Febrile prodrome: Fever of ≥101°F, 1–4 days prior to rash onset with at least prostration, headache, backache, chills, vomiting or severe abdominal pain
- Classic smallpox lesions: Deep-seated, firm/hard, round well-circumscribed vesicles or pustules; lesions may umbilicate or become confluent
- Lesions in same stage of development: On any one part of the body all lesions in same stage of development
- There are five MINOR criteria:
- Centrifugal distribution of lesions
- First lesions on the oral mucosalpalate, face, or forearms
- Patient appears toxic or moribund
- Slow evolution of lesions from macule, to papule, to vesicle (1-2 days each stage)
- Lesions on the palms and soles
- If the patient has no febrile prodrome OR has febrile prodrome AND <4 MINOR criteria, the patient’s risk of smallpox is low.
- If the patient has a history and exam suggestive of varicella, varicella testing is optional, and risk of smallpox is low.
- If the diagnosis is uncertain, test for varicella and other conditions as indicated. Risk of smallpox is low.
- If the patient has a febrile prodrome AND one other MAJOR smallpox criterion OR if the patient has a febrile prodrome AND ≥ 4 MINOR criteria, the patient’s risk of smallpox is moderate.
- Obtain Infectious Disease and/or Dermatology consultation.
- Test for Varicella and other conditions as indicated.
- If tests confirm a non-smallpox diagnosis, report results to infection control. The patient’s risk of smallpox is low.
- If no diagnosis is made after tests are completed, ensure specimen adequacy and re‑evaluate the patient. At this point, the patient’s risk of smallpox is moderate.
- If re‑evaluation does not reveal a diagnosis, you CANNOT rule out smallpox. Patient’s risk of smallpox is HIGH.
- If the patient has a febrile prodrome AND classic smallpox lesions AND lesions in the same stage of development, the patient’s risk of smallpox is HIGH.
- Obtain Infectious Disease and/or Dermatology consultation and alert local and state public health departments immediately.
- The Response team from the local and/or state public health department will advise on management and specimen collection.
- Perform variola testing at variola testing laboratory and at CDC
- If variola testing rules out smallpox, continue other diagnostic testing. Patient’s risk of smallpox is low.
- If variola is positive, then smallpox is confirmed.
- There are three MAJOR criteria:
-
